Expression and cellular localization of inducible nitric oxide synthase in lipopolysaccharide-treated rat kidneys
- PMID: 22260992
- PMCID: PMC3351238
- DOI: 10.1369/0022155411436131
Expression and cellular localization of inducible nitric oxide synthase in lipopolysaccharide-treated rat kidneys
Abstract
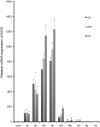
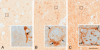
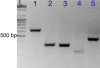
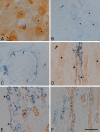
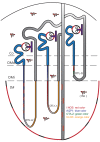
Although inducible nitric oxide synthase (iNOS) is known to play significant roles in the kidney, its renal localization has long been controversial. To resolve this issue, the authors identified iNOS-positive cell types in rat kidneys using double immunohistochemistry and confirmed iNOS positivity using enzyme histochemistry with NADPH-diaphorase (NADPH-d) and in situ RT-PCR. Adult male Sprague-Dawley rats were injected intraperitoneally with lipopolysaccharide (LPS) or saline as a control and sacrificed at various time intervals after injection. Quantitative real-time reverse transcriptase polymerase chain reaction showed that iNOS was not expressed in control kidneys but was induced in LPS-treated kidneys. iNOS immunostaining was strongest 6 to 18 hr after injection and decreased gradually to control levels by day 7. Double immunohistochemistry and NADPH-d revealed that iNOS expression was induced in the interstitial cells, glomerular parietal epithelial cells, the proximal part of the short-looped descending thin limb, the upper and middle papillary parts of the long-looped descending thin limb, some inner medullary collecting duct cells, and almost all calyceal and papillary epithelial cells. The present study determines the precise localization of iNOS in LPS-treated rat kidneys and provides an important morphological basis for examining the roles of iNOS in sepsis-induced acute kidney injury.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures





References
-
- Ahn KY, Mohaupt MG, Madsen KM, Kone BC. 1994. In situ hybridization localization of mRNA encoding inducible nitric oxide synthase in rat kidney. Am J Physiol. 267:F748-F757 - PubMed
-
- Bates TE, Loesch A, Burnstock G, Clark JB. 1996. Mitochondrial nitric oxide synthase: a ubiquitous regulator of oxidative phosphorylation? Biochem Biophys Res Commun. 218:40–44 - PubMed
-
- Cha JH, Woo SK, Han KH, Kim YH, Handler JS, Kim J, Kwon HM. 2001. Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J Am Soc Nephrol. 12:2221–2230 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

