The layered structure of coronary adventitia under mechanical load
- PMID: 22261042
- PMCID: PMC3297804
- DOI: 10.1016/j.bpj.2011.10.043
The layered structure of coronary adventitia under mechanical load
Abstract
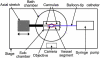
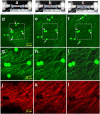
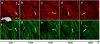
The mechanical loading-deformation relation of elastin and collagen fibril bundles is fundamental to understanding the microstructural properties of tissue. Here, we use multiphoton microscopy to obtain quantitative data of elastin and collagen fiber bundles under in situ loading of coronary adventitia. Simultaneous loading-imaging experiments on unstained fresh coronary adventitia allowed morphometric measurements of collagen and elastin fibril bundles and their individual deformation. Fiber data were analyzed at five different distension loading points (circumferential stretch ratio λ(θ) = 1.0, 1.2, 1.4, 1.6, and 1.8) at a physiological axial stretch ratio of λ(axial) = 1.3. Four fiber geometrical parameters were used to quantify the fibers: orientation angle, waviness, width, and area fraction. The results show that elastin and collagen fibers in inner adventitia form concentric densely packed fiber sheets, and the fiber orientation angle, width, and area fraction vary transmurally. The extent of fiber deformation depends on the initial orientation angle at no-distension state (λ(θ) = 1.0 and λ(axial) = 1.3). At higher distension loading, the orientation angle and waviness of fibers decrease linearly, but the width of collagen fiber is relatively constant at λ(θ) = 1.0-1.4 and then decrease linearly for λ(θ) ≥ 1.4. A decrease of the relative dispersion (SD/mean) of collagen fiber waviness suggests a heterogeneous mechanical response to loads. This study provides fundamental microstructural data for coronary artery biomechanics and we consider it seminal for structural models.
Copyright © 2011 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Rhodin J.A.G. Architecture of the vessel wall. In: Berne R.M., editor. Vol. 2. American Physiology Society; Bethesda, MD: 1979. (Handbook of Physiology). Sect. 2, 1–31.
-
- Fratzl P., Misof K., Bernstorff S. Fibrillar structure and mechanical properties of collagen. J. Struct. Biol. 1998;122:119–122. - PubMed
-
- Ottani V., Raspanti M., Ruggeri A. Collagen structure and functional implications. Micron. 2001;32:251–260. - PubMed
-
- Roach M.R., Burton A.C. The reason for the shape of the distensibility curves of arteries. Can. J. Biochem. Physiol. 1957;35:681–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

