Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences
- PMID: 22262063
- PMCID: PMC3774553
- DOI: 10.1152/ajpcell.00450.2011
Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences
Abstract
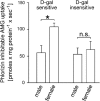
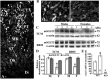
With a novel antibody against the rat Na(+)-D-glucose cotransporter SGLT2 (rSGLT2-Ab), which does not cross-react with rSGLT1 or rSGLT3, the ∼75-kDa rSGLT2 protein was localized to the brush-border membrane (BBM) of the renal proximal tubule S1 and S2 segments (S1 > S2) with female-dominant expression in adult rats, whereas rSglt2 mRNA expression was similar in both sexes. Castration of adult males increased the abundance of rSGLT2 protein; this increase was further enhanced by estradiol and prevented by testosterone treatment. In the renal BBM vesicles, the rSGLT1-independent uptake of [(14)C]-α-methyl-D-glucopyranoside was similar in females and males, suggesting functional contribution of another Na(+)-D-glucose cotransporter to glucose reabsorption. Since immunoreactivity of rSGLT2-Ab could not be detected with certainty in rat extrarenal organs, the SGLT2 protein was immunocharacterized with the same antibody in wild-type (WT) mice, with SGLT2-deficient (Sglt2 knockout) mice as negative control. In WT mice, renal localization of mSGLT2 protein was similar to that in rats, whereas in extrarenal organs neither mSGLT2 protein nor mSglt2 mRNA expression was detected. At variance to the findings in rats, the abundance of mSGLT2 protein in the mouse kidneys was male dominant, whereas the expression of mSglt2 mRNA was female dominant. Our results indicate that in rodents the expression of SGLT2 is kidney-specific and point to distinct sex and species differences in SGLT2 protein expression that cannot be explained by differences in mRNA.
Figures
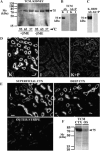
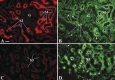
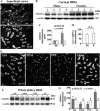
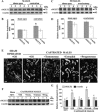



References
-
- Albertoni Borghese MF, Majowicz MP, Ortiz MC, Passalacqua MR, Sterin Speziale NB, Vidal NA. Expression and activity of SGLT2 in diabetes induced by streptozotocin: relationship with the lipid environment. Nephron Physiol 112: P45–P52, 2009 - PubMed
-
- Aljure O, Diez-Sampedro A. Functional characterization of mouse sodium/glucose transporter type 3b. Am J Physiol Cell Physiol 299: C58–C65, 2010 - PubMed
-
- Aschenbach JR, Steglich K, Gabel G, Honscha KU. Expression of mRNA for glucose transport proteins in jejunum, liver, kidney and skeletal muscle of pigs. J Physiol Biochem 65: 251–266, 2009 - PubMed
-
- Bakris GL, Fonseca VA, Sharma K, Wright EM. Renal sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Kidney Int 75: 1272–1277, 2009 - PubMed
-
- Balen D, Ljubojevic M, Breljak D, Brzica H, Zlender V, Koepsell H, Sabolic I. Revised immunolocalization of the Na+-d-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol 295: C475–C489, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

