Ezrin regulates microvillus morphogenesis by promoting distinct activities of Eps8 proteins
- PMID: 22262457
- PMCID: PMC3302735
- DOI: 10.1091/mbc.E11-07-0588
Ezrin regulates microvillus morphogenesis by promoting distinct activities of Eps8 proteins
Abstract
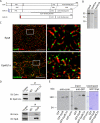
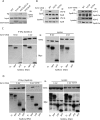
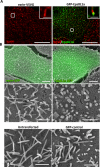
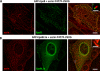
The mechanisms that regulate actin filament polymerization resulting in the morphogenesis of the brush border microvilli in epithelial cells remain unknown. Eps8, the prototype of a family of proteins capable of capping and bundling actin filaments, has been shown to bundle the microvillar actin filaments. We report that Eps8L1a, a member of the Eps8 family and a novel ezrin-interacting partner, controls microvillus length through its capping activity. Depletion of Eps8L1a leads to the formation of long microvilli, whereas its overexpression has the opposite effect. We demonstrate that ezrin differentially modulates the actin-capping and -bundling activities of Eps8 and Eps8L1a during microvillus assembly. Coexpression of ezrin with Eps8 promotes the formation of membrane ruffles and tufts of microvilli, whereas expression of ezrin and Eps8L1a induces the clustering of actin-containing structures at the cell surface. These distinct morphological changes are neither observed when a mutant of ezrin defective in its binding to Eps8/Eps8L1a is coexpressed with Eps8 or Eps8L1a nor observed when ezrin is expressed with mutants of Eps8 or Eps8L1a defective in the actin-bundling or -capping activities, respectively. Our data show a synergistic effect of ezrin and Eps8 proteins in the assembly and organization of actin microvillar filaments.
Figures





Similar articles
-
Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping.PLoS Biol. 2010 Jun 1;8(6):e1000387. doi: 10.1371/journal.pbio.1000387. PLoS Biol. 2010. PMID: 20532239 Free PMC article.
-
Comparative study of ezrin phosphorylation among different tissues: more is good; too much is bad.Am J Physiol Cell Physiol. 2008 Jul;295(1):C192-202. doi: 10.1152/ajpcell.00159.2008. Epub 2008 May 14. Am J Physiol Cell Physiol. 2008. PMID: 18480298 Free PMC article.
-
BioID2 screening identifies KIAA1671 as an EPS8 proximal factor that marks sites of microvillus growth.Mol Biol Cell. 2023 Apr 1;34(4):ar31. doi: 10.1091/mbc.E22-11-0498. Epub 2023 Feb 15. Mol Biol Cell. 2023. PMID: 36790915 Free PMC article.
-
Microvillus assembly. Not actin alone.Curr Biol. 1995 Jun 1;5(6):591-3. doi: 10.1016/s0960-9822(95)00117-5. Curr Biol. 1995. PMID: 7552163 Review.
-
The epithelial brush border Na+/H+ exchanger NHE3 associates with the actin cytoskeleton by binding to ezrin directly and via PDZ domain-containing Na+/H+ exchanger regulatory factor (NHERF) proteins.Clin Exp Pharmacol Physiol. 2008 Aug;35(8):863-71. doi: 10.1111/j.1440-1681.2008.04931.x. Epub 2008 Apr 21. Clin Exp Pharmacol Physiol. 2008. PMID: 18430067 Review.
Cited by
-
Eps8 controls Src- and FAK-dependent phenotypes in squamous carcinoma cells.J Cell Sci. 2014 Dec 15;127(Pt 24):5303-16. doi: 10.1242/jcs.157560. Epub 2014 Oct 29. J Cell Sci. 2014. PMID: 25359883 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate alters the number of attachment sites between ezrin and actin filaments: a colloidal probe study.J Biol Chem. 2014 Apr 4;289(14):9833-43. doi: 10.1074/jbc.M113.530659. Epub 2014 Feb 5. J Biol Chem. 2014. PMID: 24500715 Free PMC article.
-
Cordon Bleu serves as a platform at the basal region of microvilli, where it regulates microvillar length through its WH2 domains.Mol Biol Cell. 2014 Sep 15;25(18):2817-27. doi: 10.1091/mbc.E14-06-1131. Epub 2014 Jul 16. Mol Biol Cell. 2014. PMID: 25031432 Free PMC article.
-
Insights into the origin of metazoan filopodia and microvilli.Mol Biol Evol. 2013 Sep;30(9):2013-23. doi: 10.1093/molbev/mst110. Epub 2013 Jun 14. Mol Biol Evol. 2013. PMID: 23770652 Free PMC article.
-
A RhoA and Rnd3 cycle regulates actin reassembly during membrane blebbing.Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):E1863-71. doi: 10.1073/pnas.1600968113. Epub 2016 Mar 14. Proc Natl Acad Sci U S A. 2016. PMID: 26976596 Free PMC article.
References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Algrain M, Arpin M, Louvard D. Wizardry at the cell cortex. Cur Biol. 1993;3:451–454. - PubMed
-
- Andreoli C, Martin M, Leborgne R, Reggio H, Mangeat P. Ezrin has properties to self-associate at the plasma membrane. J Cell Sci. 1994;107:2509–2521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

