Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes
- PMID: 22262771
- PMCID: PMC3335383
- DOI: 10.1182/blood-2011-10-384826
Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes
Abstract
Cysteinyl leukotriene (cysLT) overproduction is a hallmark of aspirin-exacerbated respiratory disease (AERD), but its mechanism is poorly understood. Because adherent platelets can convert the leukocyte-derived precursor leukotriene (LT)A(4) to LTC(4), the parent cysLT, through the terminal enzyme LTC(4) synthase, we investigated the contribution of platelet-dependent transcellular cysLT production in AERD. Nasal polyps from subjects with AERD contained many extravascular platelets that colocalized with leukocytes, and the percentages of circulating neutrophils, eosinophils, and monocytes with adherent platelets were markedly higher in the blood of subjects with AERD than in aspirin-tolerant controls. Platelet-adherent subsets of leukocytes had higher expression of several adhesion markers than did platelet nonadherent subsets. Adherent platelets contributed more than half of the total LTC(4) synthase activity of peripheral blood granulocytes, and they accounted for the higher level of LTC(4) generation by activated granulocytes from subjects with AERD compared with aspirin-tolerant controls. Urinary LTE(4) levels, a measure of systemic cysLT production, correlated strongly with percentages of circulating platelet-adherent granulocytes. Because platelet adherence to leukocytes allows for both firm adhesion to endothelial cells and augmented transcellular conversion of leukotrienes, a disturbance in platelet-leukocyte interactions may be partly responsible for the respiratory tissue inflammation and the overproduction of cysLTs that characterize AERD.
Figures
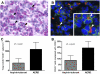


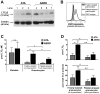

Comment in
-
Inside platelet-leukocyte cross-talk.Blood. 2012 Apr 19;119(16):3649-50. doi: 10.1182/blood-2012-02-406769. Blood. 2012. PMID: 22517871 No abstract available.
References
-
- Kasper L, Sladek K, Duplaga M, et al. Prevalence of asthma with aspirin hypersensitivity in the adult population of Poland. Allergy. 2003;58(10):1064–1066. - PubMed
-
- Reid GK, Kargman S, Vickers PJ, et al. Correlation between expression of 5-lipoxygenase-activating protein, 5-lipoxygenase, and cellular leukotriene synthesis. J Biol Chem. 1990;265(32):19818–19823. - PubMed
-
- Christie PE, Tagari P, Ford-Hutchinson AW, et al. Urinary leukotriene E4 concentrations increase after aspirin challenge in aspirin-sensitive asthmatic subjects. Am Rev Respir Dis. 1991;143(5 Pt 1):1025–1029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL117945/HL/NHLBI NIH HHS/United States
- T32 AI007306/AI/NIAID NIH HHS/United States
- U19 AI095219/AI/NIAID NIH HHS/United States
- K23 HL111113/HL/NHLBI NIH HHS/United States
- R21 AI082369/AI/NIAID NIH HHS/United States
- AI095219/AI/NIAID NIH HHS/United States
- AT002782/AT/NCCIH NIH HHS/United States
- R37 AI052353/AI/NIAID NIH HHS/United States
- P50 AT002782/AT/NCCIH NIH HHS/United States
- R01 AI052353/AI/NIAID NIH HHS/United States
- AI078908/AI/NIAID NIH HHS/United States
- P01 HL036110/HL/NHLBI NIH HHS/United States
- AI082369/AI/NIAID NIH HHS/United States
- R01 AI078908/AI/NIAID NIH HHS/United States
- HL36110/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

