Get5 carboxyl-terminal domain is a novel dimerization motif that tethers an extended Get4/Get5 complex
- PMID: 22262836
- PMCID: PMC3318709
- DOI: 10.1074/jbc.M111.333252
Get5 carboxyl-terminal domain is a novel dimerization motif that tethers an extended Get4/Get5 complex
Abstract
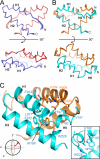
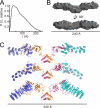
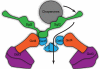
Tail-anchored trans-membrane proteins are targeted to membranes post-translationally. The proteins Get4 and Get5 form an obligate complex that catalyzes the transfer of tail-anchored proteins destined to the endoplasmic reticulum from Sgt2 to the cytosolic targeting factor Get3. Get5 forms a homodimer mediated by its carboxyl domain. We show here that a conserved motif exists within the carboxyl domain. A high resolution crystal structure and solution NMR structures of this motif reveal a novel and stable helical dimerization domain. We additionally determined a solution NMR structure of a divergent fungal homolog, and comparison of these structures allows annotation of specific stabilizing interactions. Using solution x-ray scattering and the structures of all folded domains, we present a model of the full-length Get4/Get5 complex.
Figures


References
-
- Shan S. O., Walter P. (2005) Co-translational protein targeting by the signal recognition particle. FEBS Lett. 579, 921–926 - PubMed
-
- Kutay U., Hartmann E., Rapoport T. A. (1993) A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 3, 72–75 - PubMed
-
- Borgese N., Brambillasca S., Colombo S. (2007) How tails guide tail-anchored proteins to their destinations. Curr. Opin. Cell Biol. 19, 368–375 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

