Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation
- PMID: 22262842
- PMCID: PMC3308817
- DOI: 10.1074/jbc.M111.304329
Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation
Abstract
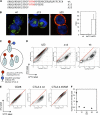
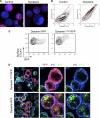
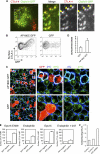
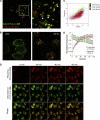
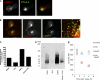
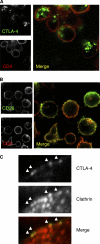
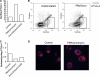
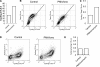
CTLA-4 is one of the most important negative regulators of the T cell immune response. However, the subcellular distribution of CTLA-4 is unusual for a receptor that interacts with cell surface transmembrane ligands in that CTLA-4 is rapidly internalized from the plasma membrane. It has been proposed that T cell activation can lead to stabilization of CTLA-4 expression at the cell surface. Here we have analyzed in detail the internalization, recycling, and degradation of CTLA-4. We demonstrate that CTLA-4 is rapidly internalized from the plasma membrane in a clathrin- and dynamin-dependent manner driven by the well characterized YVKM trafficking motif. Furthermore, we show that once internalized, CTLA-4 co-localizes with markers of recycling endosomes and is recycled to the plasma membrane. Although we observed limited co-localization of CTLA-4 with lysosomal markers, CTLA-4 was nonetheless degraded in a manner inhibited by lysosomal blockade. T cell activation stimulated mobilization of CTLA-4, as judged by an increase in cell surface expression; however, this pool of CTLA-4 continued to endocytose and was not stably retained at the cell surface. These data support a model of trafficking whereby CTLA-4 is constitutively internalized in a ligand-independent manner undergoing both recycling and degradation. Stimulation of T cells increases CTLA-4 turnover at the plasma membrane; however, CTLA-4 endocytosis continues and is not stabilized during activation of human T cells. These findings emphasize the importance of clathrin-mediated endocytosis in regulating CTLA-4 trafficking throughout T cell activation.
Figures
References
-
- Tivol E. A., Borriello F., Schweitzer A. N., Lynch W. P., Bluestone J. A., Sharpe A. H. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 - PubMed
-
- Waterhouse P., Penninger J. M., Timms E., Wakeham A., Shahinian A., Lee K. P., Thompson C. B., Griesser H., Mak T. W. (1995) Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 - PubMed
-
- Teft W. A., Kirchhof M. G., Madrenas J. (2006) A molecular perspective of CTLA-4 function. Annu. Rev. Immunol. 24, 65–97 - PubMed
-
- Rudd C. E. (2008) The reverse stop-signal model for CTLA4 function. Nat. Rev. Immunol. 8, 153–160 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/D011000/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 19364/VAC_/Versus Arthritis/United Kingdom
- 19364/ARC_/Arthritis Research UK/United Kingdom
- 092578/WT_/Wellcome Trust/United Kingdom
- BB/H013598/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

