Serine/threonine phosphatase (SP-STP), secreted from Streptococcus pyogenes, is a pro-apoptotic protein
- PMID: 22262847
- PMCID: PMC3308748
- DOI: 10.1074/jbc.M111.316554
Serine/threonine phosphatase (SP-STP), secreted from Streptococcus pyogenes, is a pro-apoptotic protein
Abstract
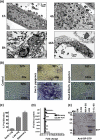
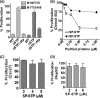
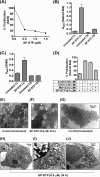
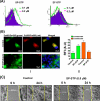
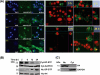
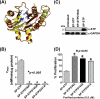
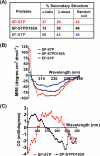
This investigation illustrates an important property of eukaryote-type serine/threonine phosphatase (SP-STP) of group A Streptococcus (GAS) in causing programmed cell death of human pharyngeal cells. The secretory nature of SP-STP, its elevated expression in the intracellular GAS, and the ability of wild-type GAS but not the GAS mutant devoid of SP-STP to cause apoptosis of the host cell both in vitro and in vivo suggest that GAS deploys SP-STP as an important virulence determinant to exploit host cell machinery for its own advantage during infection. The exogenously added SP-STP is able to enter the cytoplasm and subsequently traverses into the nucleus in a temporal fashion to cause apoptosis of the pharyngeal cells. The programmed cell death induced by SP-STP, which requires active transcription and de novo protein synthesis, is also caspase-dependent. Furthermore, the entry of SP-STP into the cytoplasm is dependent on its secondary structure as the catalytically inactive SP-STP with an altered structure is unable to internalize and cause apoptosis. The ectopically expressed wild-type SP-STP was found to be in the nucleus and conferred apoptosis of Detroit 562 pharyngeal cells. However, the catalytically inactive SP-STP was unable to cause apoptosis even when intracellularly expressed. The ability of SP-STP to activate pro-apoptotic signaling cascades both in the cytoplasm and in the nucleus resulted in mitochondrial dysfunctioning and perturbation in the phosphorylation status of histones in the nucleus. SP-STP thus not only functions as a virulence regulator but also as an important factor responsible for host-related pathogenesis.
Figures



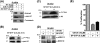

References
-
- Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. (2005) The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 - PubMed
-
- Olsen R. J., Shelburne S. A., Musser J. M. (2009) Molecular mechanisms underlying group A streptococcal pathogenesis. Cell. Microbiol. 11, 1–12 - PubMed
-
- Virtaneva K., Porcella S. F., Graham M. R., Ireland R. M., Johnson C. A., Ricklefs S. M., Babar I., Parkins L. D., Romero R. A., Corn G. J., Gardner D. J., Bailey J. R., Parnell M. J., Musser J. M. (2005) Longitudinal analysis of the group A Streptococcus transcriptome in experimental pharyngitis in cynomolgus macaques. Proc. Natl. Acad. Sci. U.S.A. 102, 9014–9019 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

