Cervicolumbar coordination in mammalian quadrupedal locomotion: role of spinal thoracic circuitry and limb sensory inputs
- PMID: 22262893
- PMCID: PMC6621141
- DOI: 10.1523/JNEUROSCI.4640-11.2012
Cervicolumbar coordination in mammalian quadrupedal locomotion: role of spinal thoracic circuitry and limb sensory inputs
Abstract
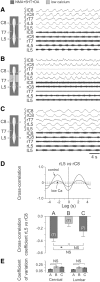
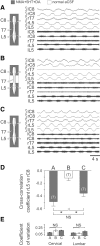
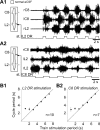
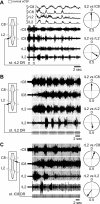
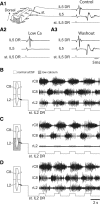
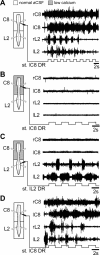
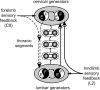
Effective quadrupedal locomotion requires a close coordination between the spatially distant central pattern generators (CPGs) controlling forelimb and hindlimb movements. Using isolated preparations of the neonatal rat spinal cord, we explore the role of intervening thoracic circuitry in cervicolumbar CPG coordination and the contribution to this remote coupling of limb somatosensory inputs. In preparations activated with bath-applied N-methyl-D,L-aspartate, serotonin, and dopamine, the coordination between locomotor-related bursts recorded in cervical and lumbar ventral roots was substantially weakened, although not abolished, when the thoracic segments were selectively withheld from neurochemical stimulation or were exposed to a low Ca(2+) solution to block synaptic transmission. Moreover, cervicolumbar CPG coordination was reduced after a thoracic midsagittal section, suggesting that cross-cord projections participate in the anteroposterior coupling. In quiescent preparations, either cyclic or tonic electrical stimulation of low-threshold afferent pathways in C8 or L2 dorsal roots (DRs) could elicit coordinated ventral root bursting at both cervical and lumbar levels via an activation of the underlying CPG networks. When lumbar rhythmogenesis was prevented by local synaptic transmission blockade, L2 DR stimulation could still drive left-right alternating cervical bursting in preparations otherwise exposed to normal bathing medium. In contrast, when the cervical generators were selectively blocked, C8 DR stimulation was unable to activate the lumbar CPGs. Thus, in the newborn rat, anteroposterior limb coordination relies on active burst generation within midcord thoracic circuitry that additionally conveys ascending and weaker descending coupling influences of distant limb proprioceptive inputs to the cervical and lumbar generators, respectively.
Figures



Similar articles
-
Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat.Eur J Neurosci. 2001 Nov;14(10):1727-38. doi: 10.1046/j.0953-816x.2001.01794.x. Eur J Neurosci. 2001. PMID: 11860467
-
Locomotor rhythmogenesis in the isolated rat spinal cord: a phase-coupled set of symmetrical flexion extension oscillators.J Physiol. 2007 Aug 15;583(Pt 1):115-28. doi: 10.1113/jphysiol.2007.133413. Epub 2007 Jun 14. J Physiol. 2007. PMID: 17569737 Free PMC article.
-
Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion.J Neurosci. 2005 Jun 22;25(25):6025-35. doi: 10.1523/JNEUROSCI.0696-05.2005. J Neurosci. 2005. PMID: 15976092 Free PMC article.
-
Tuning and playing a motor rhythm: how metabotropic glutamate receptors orchestrate generation of motor patterns in the mammalian central nervous system.J Physiol. 2006 Apr 15;572(Pt 2):323-34. doi: 10.1113/jphysiol.2005.100610. Epub 2006 Feb 9. J Physiol. 2006. PMID: 16469790 Free PMC article. Review.
-
Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord.Front Neural Circuits. 2014 Aug 28;8:102. doi: 10.3389/fncir.2014.00102. eCollection 2014. Front Neural Circuits. 2014. PMID: 25221477 Free PMC article. Review.
Cited by
-
Silencing long ascending propriospinal neurons after spinal cord injury improves hindlimb stepping in the adult rat.Elife. 2021 Dec 2;10:e70058. doi: 10.7554/eLife.70058. Elife. 2021. PMID: 34854375 Free PMC article.
-
Stabilization of cat paw trajectory during locomotion.J Neurophysiol. 2014 Sep 15;112(6):1376-91. doi: 10.1152/jn.00663.2013. Epub 2014 Jun 3. J Neurophysiol. 2014. PMID: 24899676 Free PMC article.
-
Dense distributed processing in a hindlimb scratch motor network.J Neurosci. 2014 Aug 6;34(32):10756-64. doi: 10.1523/JNEUROSCI.1079-14.2014. J Neurosci. 2014. PMID: 25100606 Free PMC article.
-
Increasing cognitive load attenuates right arm swing in healthy human walking.R Soc Open Sci. 2017 Jan 25;4(1):160993. doi: 10.1098/rsos.160993. eCollection 2017 Jan. R Soc Open Sci. 2017. PMID: 28280596 Free PMC article.
-
A novel device for studying weight supported, quadrupedal overground locomotion in spinal cord injured rats.J Neurosci Methods. 2015 May 15;246:134-41. doi: 10.1016/j.jneumeth.2015.03.015. Epub 2015 Mar 18. J Neurosci Methods. 2015. PMID: 25794460 Free PMC article.
References
-
- Baldissera F, Cavallari P, Leocani L. Cyclic modulation of the H-reflex in a wrist flexor during rhythmic flexion-extension movements of the ipsilateral foot. Exp Brain Res. 1998;118:427–430. - PubMed
-
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Barriere G, Mellen N, Cazalets JR. Neuromodulation of the locomotor network by dopamine in the isolated spinal cord of newborn rat. Eur J Neurosci. 2004;19:1325–1335. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
