Overcoming the "oxidant problem": strategies to use O2 as the oxidant in organometallic C-H oxidation reactions catalyzed by Pd (and Cu)
- PMID: 22263575
- PMCID: PMC3355522
- DOI: 10.1021/ar2002045
Overcoming the "oxidant problem": strategies to use O2 as the oxidant in organometallic C-H oxidation reactions catalyzed by Pd (and Cu)
Abstract
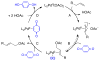
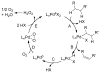
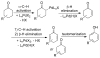
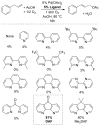
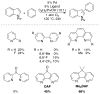
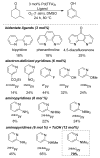
Oxidation reactions are key transformations in organic chemistry because they can increase chemical complexity and incorporate heteroatom substituents into carbon-based molecules. This principle is manifested in the conversion of petrochemical feedstocks into commodity chemicals and in the synthesis of fine chemicals, pharmaceuticals, and other complex organic molecules. The utility and function of these molecules correlate directly with the presence and specific placement of oxygen and nitrogen heteroatoms and other functional groups within the molecules. Methods for selective oxidation of C-H bonds have expanded significantly over the past decade, and their role in the synthesis of organic chemicals will continue to increase. Our group's contributions to this field are linked to our broader interest in the development and mechanistic understanding of aerobic oxidation reactions. Molecular oxygen (O(2)) is the ideal oxidant. Its low cost and lack of toxic byproducts make it a highly appealing reagent that can address key "green chemistry" priorities in industry. With strong economic and environmental incentives to use O(2), the commmodity chemicals industry often uses aerobic oxidation reactions. In contrast, O(2) is seldom used to prepare more-complex smaller-volume chemicals, a limitation that reflects, in part, the limited synthetic scope and utility of existing aerobic reactions. Pd-catalyzed reactions represent some of the most versatile methods for selective C-H oxidation, but they often require stoichiometric transition-metal or organic oxidants, such as Cu(II), Ag(I), or benzoquinone. This Account describes recent strategies that we have identified to use O(2) as the oxidant in these reactions. In Pd-catalyzed C-H oxidation reactions that form carbon-heteroatom bonds, the stoichiometric oxidant is often needed to promote difficult reductive elimination steps in the catalytic mechanism. To address this challenge, we have identified new ancillary ligands for Pd that promote reductive elimination, or replaced Pd with a Cu catalyst that undergoes facile reductive elimination from a Cu(III) intermediate. Both strategies have enabled O(2) to be used as the sole stoichiometric oxidant in the catalytic reactions. C-H oxidation reactions that form the product via β-hydride or C-C reductive elimination steps tend to be more amenable to the use of O(2). The use of new ancillary ligands has also overcome some of the limitations in these methods. Mechanistic studies are providing insights into some (but not yet all) of these advances in catalytic reactivity.
Figures

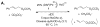
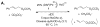












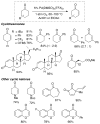
References
-
-
For leading references, see the February 2010 and March 2011 issues of Chem. Rev. and the April 2011 issue of Chem. Soc. Rev.
-
-
- Gol’dshleger NF, Es’kova VV, Shteinman AA, Shilov AE. Reactions of Alkanes in Solutions of Chloride Complexes of Platinum. Zh Fiz Khim (Engl Transl) 1972;46:785–786.
-
- Stahl SS, Labinger JA, Bercaw JE. Homogeneous Oxidation of Alkanes by Electrophilic Late Transition Metals. Angew Chem Int Ed. 1998;37:2181–2192. - PubMed
-
- Dick AR, Hull KL, Sanford MS. A Highly Selective Catalytic Method for the Oxidative Functionalization of C-H Bonds. J Am Chem Soc. 2004;126:2300–2301. - PubMed
- Giri R, Chen X, Yu JQ. Palladium-Catalyzed Asymmetric Iodination of Unactivated C-H Bonds under Mild Conditions. Angew Chem Int Ed. 2005;44:2112–2115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

