Anti-angiogenic properties of ADAMTS-4 in vitro
- PMID: 22264287
- PMCID: PMC3311023
- DOI: 10.1111/j.1365-2613.2011.00802.x
Anti-angiogenic properties of ADAMTS-4 in vitro
Abstract
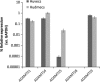
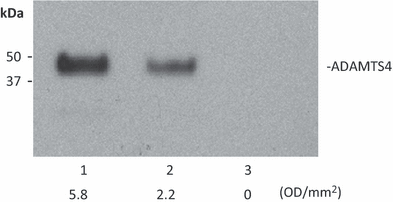
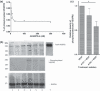
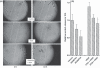
Angiogenesis is an indispensable mechanism in development and in many pathologies, including cancer, synovitis and aberrant wound healing. Many angiogenic stimulators and inhibitors have been investigated, and some have progressed to the clinic. A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) is a group of multifunctional proteinases. ADAMTS-1 and ADAMTS-8 have been reported to be anti-angiogenic. Here, we provide evidence that ADAMTS-4, like ADAMTS-1, is expressed by endothelial cells and binds to vascular endothelial groth factor (VEGF). Moreover, ADAMTS-4 inhibited human dermal microvascular endothelial cells (HuDMEC) VEGF-stimulated VEGF receptor (R) R2 phosphorylation, differentiation and migration, suggesting that ADAMTS-4 may be a novel anti-angiogenic molecule.
© 2012 The Authors. International Journal of Experimental Pathology © 2012 International Journal of Experimental Pathology.
Figures

Similar articles
-
The roles of ADAMTS in angiogenesis and cancer.Tumour Biol. 2015 Jun;36(6):4039-51. doi: 10.1007/s13277-015-3461-8. Epub 2015 Apr 28. Tumour Biol. 2015. PMID: 25916206 Review.
-
Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork.Invest Ophthalmol Vis Sci. 2009 Dec;50(12):5769-77. doi: 10.1167/iovs.09-3673. Epub 2009 Jun 24. Invest Ophthalmol Vis Sci. 2009. PMID: 19553617 Free PMC article.
-
ADAMTS proteoglycanases in the physiological and pathological central nervous system.J Neuroinflammation. 2013 Oct 31;10:133. doi: 10.1186/1742-2094-10-133. J Neuroinflammation. 2013. PMID: 24176075 Free PMC article. Review.
-
ADAMTS-2 functions as anti-angiogenic and anti-tumoral molecule independently of its catalytic activity.Cell Mol Life Sci. 2010 Dec;67(24):4213-32. doi: 10.1007/s00018-010-0431-6. Epub 2010 Jun 24. Cell Mol Life Sci. 2010. PMID: 20574651 Free PMC article.
-
Regulation of ADAMTS-1, -4 and -5 expression in human macrophages: differential regulation by key cytokines implicated in atherosclerosis and novel synergism between TL1A and IL-17.Cytokine. 2013 Oct;64(1):234-42. doi: 10.1016/j.cyto.2013.06.315. Epub 2013 Jul 13. Cytokine. 2013. PMID: 23859810 Free PMC article.
Cited by
-
The roles of ADAMTS in angiogenesis and cancer.Tumour Biol. 2015 Jun;36(6):4039-51. doi: 10.1007/s13277-015-3461-8. Epub 2015 Apr 28. Tumour Biol. 2015. PMID: 25916206 Review.
-
ADAMTS proteases in vascular biology.Matrix Biol. 2015 May-Jul;44-46:38-45. doi: 10.1016/j.matbio.2015.02.004. Epub 2015 Feb 17. Matrix Biol. 2015. PMID: 25698314 Free PMC article. Review.
-
ADAMTS-4 and biglycan are expressed at high levels and co-localize to podosomes during endothelial cell tubulogenesis in vitro.J Histochem Cytochem. 2014 Jan;62(1):34-49. doi: 10.1369/0022155413507727. Epub 2013 Sep 18. J Histochem Cytochem. 2014. PMID: 24051360 Free PMC article.
-
Emerging Roles of ADAMTSs in Angiogenesis and Cancer.Cancers (Basel). 2012 Nov 29;4(4):1252-99. doi: 10.3390/cancers4041252. Cancers (Basel). 2012. PMID: 24213506 Free PMC article.
-
Biosynthesis and expression of a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats-15: a novel versican-cleaving proteoglycanase.J Biol Chem. 2013 Dec 27;288(52):37267-76. doi: 10.1074/jbc.M112.418624. Epub 2013 Nov 12. J Biol Chem. 2013. PMID: 24220035 Free PMC article.
References
-
- Donovan D, Brown NJ, Bishop ET, Lewis CE. Comparison of three in vitro angiogenesis assays with capillaries formed in vivo. Angiogenesis. 2001;4:113–121. - PubMed
-
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur. Cytokine Netw. 2009;20:158–163. - PubMed
-
- Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. - PubMed
-
- Iruela-Arispe ML, Lombardo M, Krutzsch HC, Lawler J, Roberts DD. Inhibition of angiogenesis by thrombospondin-1 is mediated by 2 independent regions within the type 1 repeats. Circulation. 1999;100:1423–1431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

