Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions
- PMID: 22264356
- PMCID: PMC3292819
- DOI: 10.1186/1749-8104-7-4
Sonic hedgehog lineage in the mouse hypothalamus: from progenitor domains to hypothalamic regions
Abstract
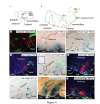
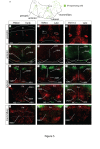
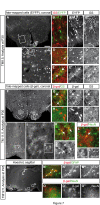
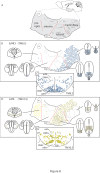
Background: The hypothalamus is a brain region with essential functions for homeostasis and energy metabolism, and alterations of its development can contribute to pathological conditions in the adult, like hypertension, diabetes or obesity. However, due to the anatomical complexity of the hypothalamus, its development is not well understood. Sonic hedgehog (Shh) is a key developmental regulator gene expressed in a dynamic pattern in hypothalamic progenitor cells. To obtain insight into hypothalamic organization, we used genetic inducible fate mapping (GIFM) to map the lineages derived from Shh-expressing progenitor domains onto the four rostrocaudally arranged hypothalamic regions: preoptic, anterior, tuberal and mammillary.
Results: Shh-expressing progenitors labeled at an early stage (before embryonic day (E)9.5) contribute neurons and astrocytes to a large caudal area including the mammillary and posterior tuberal regions as well as tanycytes (specialized median eminence glia). Progenitors labeled at later stages (after E9.5) give rise to neurons and astrocytes of the entire tuberal region and in particular the ventromedial nucleus, but not to cells in the mammillary region and median eminence. At this stage, an additional Shh-expressing domain appears in the preoptic area and contributes mostly astrocytes to the hypothalamus. Shh-expressing progenitors do not contribute to the anterior region at any stage. Finally, we show a gradual shift from neurogenesis to gliogenesis, so that progenitors expressing Shh after E12.5 generate almost exclusively hypothalamic astrocytes.
Conclusions: We define a fate map of the hypothalamus, based on the dynamic expression of Shh in the hypothalamic progenitor zones. We provide evidence that the large neurogenic Shh-expressing progenitor domains of the ventral diencephalon are continuous with those of the midbrain. We demonstrate that the four classical transverse zones of the hypothalamus have clearly defined progenitor domains and that there is little or no cell mixing between the tuberal and anterior or the preoptic and anterior hypothalamus. Finally, we show that, in the tuberal hypothalamus, neurons destined for every mediolateral level are produced during a period of days, in conflict with the current 'three-wave' model of hypothalamic neurogenesis. Our work sets the stage for a deeper developmental analysis of this complex and important brain region.
Figures




References
-
- Saper CB. Staying awake for dinner: hypothalamic integration of sleep, feeding, and circadian rhythms. Prog Brain Res. 2006;153:243–252. - PubMed
-
- Simerly RB. In: The Rat Nervous System. Paxinos G, editor. Amsterdam: Elsevier; 2004. pp. 335–368.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

