Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific Immunoglobulin-E
- PMID: 22264407
- PMCID: PMC3398323
- DOI: 10.1186/1710-1492-8-1
Basophil activation test compared to skin prick test and fluorescence enzyme immunoassay for aeroallergen-specific Immunoglobulin-E
Abstract
Background: Skin prick test (SPT) and fluorescence enzyme immunoassay (FEIA) are widely used for the diagnosis of Immunoglobulin-E (IgE)-mediated allergic disease. Basophil activation test (BAT) could obviate disadvantages of SPT and FEIA. However, it is not known whether BAT gives similar results as SPT or FEIA for aeroallergens.
Objectives: In this study, we compared the results of SPT, BAT and FEIA for different aeroallergens.
Methods: We performed BAT, SPT and FEIA in 41 atopic subjects (symptomatic and with positive SPT for at least 1 of 9 common aeroallergens) and 31 non-atopic subjects (asymptomatic and with negative SPT).
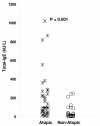
Results: Correlations between SPT and BAT, SPT and FEIA, and BAT and FEIA results were statistically significant but imperfect. Using SPT as the "gold standard", BAT and FEIA were similar in sensitivity. However, BAT had lower specificity than FEIA. False positive (BATposSPTneg) results were frequent in those atopic subjects who were allergic by SPT to a different allergen and rare in non-atopic subjects. The false positivity in atopic subjects was due in part to high levels of serum Total-IgE (T-IgE) levels in atopic individuals that lead to basophil activation upon staining with fluorochrome-labeled anti-IgE.
Conclusion: As an alternative to SPT in persons allergic to aeroallergens, BAT in its present form is useful for distinguishing atopic from non-atopic persons. However, BAT in its present form is less specific than FEIA when determining the allergen which a patient is allergic to. This is due to IgE staining-induced activation of atopic person's basophils and/or nonspecific hyperreactivity of atopic person's basophils.
Figures





Similar articles
-
CD63 expression on basophils as a tool for the diagnosis of pollen-associated food allergy: sensitivity and specificity.Clin Exp Allergy. 2003 May;33(5):607-14. doi: 10.1046/j.1365-2222.2003.01660.x. Clin Exp Allergy. 2003. PMID: 12752589
-
Evaluation of basophil activation test in suspected food hypersensitivity.Cytometry B Clin Cytom. 2017 Jul;92(4):279-285. doi: 10.1002/cyto.b.21264. Epub 2015 Jul 17. Cytometry B Clin Cytom. 2017. PMID: 26184676
-
Analysis of basophil activation by flow cytometry in pediatric house dust mite allergy.Pediatr Allergy Immunol. 2008 Jun;19(4):342-7. doi: 10.1111/j.1399-3038.2007.00656.x. Epub 2008 Feb 11. Pediatr Allergy Immunol. 2008. PMID: 18266832 Clinical Trial.
-
[Basophil activation test--a practical approach to diagnosis of common respiratory allergy].Przegl Lek. 2015;72(12):725-30. Przegl Lek. 2015. PMID: 27024948 Polish.
-
Enhancing allergy diagnosis: mass spectrometry as a complementary technique to the basophil activation test.Front Allergy. 2025 May 30;6:1568670. doi: 10.3389/falgy.2025.1568670. eCollection 2025. Front Allergy. 2025. PMID: 40519814 Free PMC article. Review.
Cited by
-
Immunotherapy in allergy and cellular tests: state of art.Hum Vaccin Immunother. 2014;10(6):1595-610. doi: 10.4161/hv.28592. Epub 2014 May 2. Hum Vaccin Immunother. 2014. PMID: 24717453 Free PMC article. Review.
-
Immediate Hypersensitivity Reactions Induced by COVID-19 Vaccines: Current Trends, Potential Mechanisms and Prevention Strategies.Biomedicines. 2022 May 28;10(6):1260. doi: 10.3390/biomedicines10061260. Biomedicines. 2022. PMID: 35740283 Free PMC article. Review.
-
Emerging role of human basophil biology in health and disease.Curr Allergy Asthma Rep. 2014 Jan;14(1):408. doi: 10.1007/s11882-013-0408-2. Curr Allergy Asthma Rep. 2014. PMID: 24346805 Free PMC article. Review.
-
Precision medicine allergy immunoassay methods for assessing immunoglobulin E sensitization to aeroallergen molecules.World J Methodol. 2018 Nov 29;8(3):17-36. doi: 10.5662/wjm.v8.i3.17. eCollection 2018 Nov 29. World J Methodol. 2018. PMID: 30519536 Free PMC article.
-
A designer cell-based histamine-specific human allergy profiler.Nat Commun. 2014 Aug 5;5:4408. doi: 10.1038/ncomms5408. Nat Commun. 2014. PMID: 25093291 Free PMC article.
References
-
- Liccardi G, D'Amato D, Canonica GW, Salzillo A, Piccolo A, Passalacqua G. Systemic reactions from skin testing: literature review. J Investig Allergol Clin Immunol. 2006;16:75–8. - PubMed
-
- Ostergaard PA, Ebbesen F, Nolte H, Skov PS. Basophil histamine release in the diagnosis of house dust mite and dander allergy of asthmatic children. Comparison between prick test, RAST, basophil histamine release and bronchial provocation. Allergy. 1990;45:231–5. doi: 10.1111/j.1398-9995.1990.tb00488.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

