β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner
- PMID: 22264443
- PMCID: PMC3319102
- DOI: 10.1016/j.biopsych.2011.11.027
β-arrestin2 regulates cannabinoid CB1 receptor signaling and adaptation in a central nervous system region-dependent manner
Abstract
Background: Cannabinoid CB(1) receptors (CB(1)Rs) mediate the effects of ▵(9)-tetrahydrocannabinol (THC), the psychoactive component in marijuana. Repeated THC administration produces tolerance and dependence, which limit therapeutic development. Moreover, THC produces motor and psychoactive side effects. β-arrestin2 mediates receptor desensitization, internalization, and signaling, but its role in these CB(1)R effects and receptor regulation is unclear.
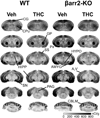
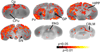
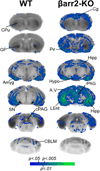
Methods: CB(1)R signaling and behaviors (antinociception, hypothermia, catalepsy) were assessed in β-arrestin2-knockout (βarr2-KO) and wild-type mice after THC administration. Cannabinoid-stimulated [(35)S]GTPγS and [(3)H]ligand autoradiography were assessed by statistical parametric mapping and region-of-interest analysis.
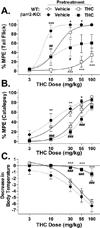
Results: β-arrestin2 deletion increased CB(1)R-mediated G-protein activity in subregions of the cortex but did not affect CB(1)R binding, in vehicle-treated mice. βarr2-KO mice exhibited enhanced acute THC-mediated antinociception and hypothermia, with no difference in catalepsy. After repeated THC administration, βarr2-KO mice showed reduced CB(1)R desensitization and/or downregulation in cerebellum, caudal periaqueductal gray, and spinal cord and attenuated tolerance to THC-mediated antinociception. In contrast, greater desensitization was found in hypothalamus, cortex, globus pallidus, and substantia nigra of βarr2-KO compared with wild-type mice. Enhanced tolerance to THC-induced catalepsy was observed in βarr2-KO mice.
Conclusions: β-arrestin2 regulation of CB(1)R signaling following acute and repeated THC administration was region-specific, and results suggest that multiple, overlapping mechanisms regulate CB(1)Rs. The observations that βarr2-KO mice display enhanced antinociceptive responses to acute THC and decreased tolerance to the antinociceptive effects of the drug, yet enhanced tolerance to catalepsy, suggest that development of cannabinoid drugs that minimize CB(1)R interactions with β-arrestin2 might produce improved cannabinoid analgesics with reduced motor suppression.
Copyright © 2012 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
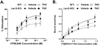


Similar articles
-
FAAH-/- mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration.Neuropsychopharmacology. 2010 Jul;35(8):1775-87. doi: 10.1038/npp.2010.44. Epub 2010 Mar 31. Neuropsychopharmacology. 2010. PMID: 20357755 Free PMC article.
-
Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors.Eur J Pharmacol. 2007 Oct 1;571(2-3):129-37. doi: 10.1016/j.ejphar.2007.06.001. Epub 2007 Jun 12. Eur J Pharmacol. 2007. PMID: 17603035 Free PMC article.
-
Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice.J Pharmacol Exp Ther. 2002 Oct;303(1):36-44. doi: 10.1124/jpet.102.035618. J Pharmacol Exp Ther. 2002. PMID: 12235230
-
Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids.Crit Rev Neurobiol. 2003;15(2):91-119. doi: 10.1615/critrevneurobiol.v15.i2.10. Crit Rev Neurobiol. 2003. PMID: 14977366 Review.
-
Brain regional differences in CB1 receptor adaptation and regulation of transcription.Life Sci. 2013 Mar 19;92(8-9):446-52. doi: 10.1016/j.lfs.2012.08.023. Epub 2012 Aug 24. Life Sci. 2013. PMID: 22940268 Free PMC article. Review.
Cited by
-
Lack of hippocampal CB1 receptor desensitization by Δ(9)-tetrahydrocannabinol in aged mice and by low doses of JZL 184.Naunyn Schmiedebergs Arch Pharmacol. 2016 Jun;389(6):603-12. doi: 10.1007/s00210-016-1226-6. Epub 2016 Mar 17. Naunyn Schmiedebergs Arch Pharmacol. 2016. PMID: 26984820
-
Dissociation between the anti-allodynic effects of fingolimod (FTY720) and desensitization of S1P1 receptor-mediated G-protein activation in a mouse model of sciatic nerve injury.Neuropharmacology. 2024 Dec 15;261:110165. doi: 10.1016/j.neuropharm.2024.110165. Epub 2024 Sep 18. Neuropharmacology. 2024. PMID: 39303855
-
Structural Insights into CB1 Receptor Biased Signaling.Int J Mol Sci. 2019 Apr 13;20(8):1837. doi: 10.3390/ijms20081837. Int J Mol Sci. 2019. PMID: 31013934 Free PMC article. Review.
-
Cannabinoid Receptor Interacting Protein 1a Competition with β-Arrestin for CB1 Receptor Binding Sites.Mol Pharmacol. 2017 Feb;91(2):75-86. doi: 10.1124/mol.116.104638. Epub 2016 Nov 28. Mol Pharmacol. 2017. PMID: 27895162 Free PMC article.
-
Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases.Int J Mol Sci. 2020 Oct 17;21(20):7693. doi: 10.3390/ijms21207693. Int J Mol Sci. 2020. PMID: 33080916 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

