Expanding the morphogenetic repertoire: perspectives from the Drosophila egg
- PMID: 22264728
- PMCID: PMC3266552
- DOI: 10.1016/j.devcel.2011.12.003
Expanding the morphogenetic repertoire: perspectives from the Drosophila egg
Abstract
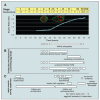
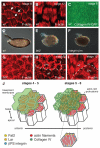
Tissue and organ architectures are incredibly diverse, yet our knowledge of the morphogenetic behaviors that generate them is relatively limited. Recent studies have revealed unexpected mechanisms that drive axis elongation in the Drosophila egg, including an unconventional planar polarity signaling pathway, a distinctive type of morphogenetic movement termed "global tissue rotation," a molecular corset-like role of extracellular matrix, and oscillating basal cellular contractions. We review here what is known about Drosophila egg elongation, compare it to other instances of morphogenesis, and highlight several issues of general developmental relevance.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Adams DS, Keller R, Koehl MAR. The Mechanics of Notochord Elongation, Straightening and Stiffening in the Embryo of Xenopus-Laevis. Development. 1990;110:115–130. - PubMed
-
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, Eaton S. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. - PubMed
-
- Axelrod JD. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol. 2009;20:964–971. - PubMed
-
- Baskin TI. Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol. 2005;21:203–222. - PubMed
-
- Bateman J, Reddy R, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–1327. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

