Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells
- PMID: 22264730
- PMCID: PMC3266520
- DOI: 10.1016/j.devcel.2011.10.030
Phosphoinositide signaling regulates the exocyst complex and polarized integrin trafficking in directionally migrating cells
Abstract
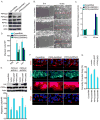
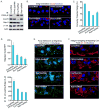
Polarized delivery of signaling and adhesion molecules to the leading edge is required for directional migration of cells. Here, we describe a role for the PIP(2)-synthesizing enzyme, PIPKIγi2, in regulation of exocyst complex control of cell polarity and polarized integrin trafficking during migration. Loss of PIPKIγi2 impaired directional migration, formation of cell polarity, and integrin trafficking to the leading edge. Upon initiation of directional migration, PIPKIγi2 via PIP(2) generation controls the integration of the exocyst complex into an integrin-containing trafficking compartment that requires the talin-binding ability of PIPKIγi2, and talin for integrin recruitment to the leading edge. A PIP(2) requirement is further emphasized by inhibition of PIPKIγi2-regulated directional migration by an Exo70 mutant deficient in PIP(2) binding. These results reveal how phosphoinositide generation orchestrates polarized trafficking of integrin in coordination with talin that links integrins to the actin cytoskeleton, processes that are required for directional migration.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures





References
-
- Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem. 1999;274:9907–9910. - PubMed
-
- Bairstow SF, Ling K, Su X, Firestone AJ, Carbonara C, Anderson RA. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J Biol Chem. 2006;281:20632–20642. - PubMed
-
- Bretscher MS. Endocytosis: relation to capping and cell locomotion. Science. 1984;224:681–686. - PubMed
-
- Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

