RIP3 finds partners in crime
- PMID: 22265396
- PMCID: PMC3571655
- DOI: 10.1016/j.cell.2011.12.020
RIP3 finds partners in crime
Abstract
Programmed necrosis has long been recognized as a crucial component of animal development; however, the signaling pathway beyond the protein kinases RIP1 and RIP3 remains largely unknown. Sun et al. and Wang et al. now identify critical factors downstream of RIP1 and RIP3 in programmed necrosis, extending our understanding of this form of cell death.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
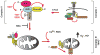
Comment on
-
Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase.Cell. 2012 Jan 20;148(1-2):213-27. doi: 10.1016/j.cell.2011.11.031. Cell. 2012. PMID: 22265413
-
The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways.Cell. 2012 Jan 20;148(1-2):228-43. doi: 10.1016/j.cell.2011.11.030. Cell. 2012. PMID: 22265414
References
-
- He SD, Wang L, Miao L, Wang T, Du FH, Zhao LP, Wang XD. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-alpha. Cell. 2009;137:1100–1111. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

