Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock
- PMID: 22265401
- PMCID: PMC3336960
- DOI: 10.1016/j.cell.2012.01.003
Aging, rejuvenation, and epigenetic reprogramming: resetting the aging clock
Abstract
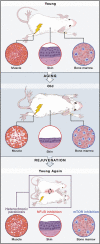
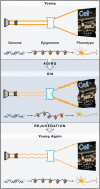
The underlying cause of aging remains one of the central mysteries of biology. Recent studies in several different systems suggest that not only may the rate of aging be modified by environmental and genetic factors, but also that the aging clock can be reversed, restoring characteristics of youthfulness to aged cells and tissues. This Review focuses on the emerging biology of rejuvenation through the lens of epigenetic reprogramming. By defining youthfulness and senescence as epigenetic states, a framework for asking new questions about the aging process emerges.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

References
-
- Bahar R, Hartmann CH, Rodriguez KA, Denny AD, Busuttil RA, Dollé ME, Calder RB, Chisholm GB, Pollock BH, Klein CA, Vijg J. Increased cell-to-cell variation in gene expression in ageing mouse heart. Nature. 2006;441:1011–1014. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

