Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell
- PMID: 22265412
- PMCID: PMC3268671
- DOI: 10.1016/j.cell.2011.10.051
Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell
Abstract
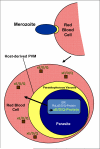
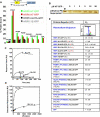
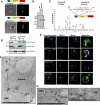
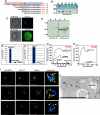
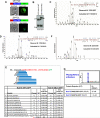
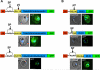
Hundreds of effector proteins of the human malaria parasite Plasmodium falciparum constitute a "secretome" carrying a host-targeting (HT) signal, which predicts their export from the intracellular pathogen into the surrounding erythrocyte. Cleavage of the HT signal by a parasite endoplasmic reticulum (ER) protease, plasmepsin V, is the proposed export mechanism. Here, we show that the HT signal facilitates export by recognition of the lipid phosphatidylinositol-3-phosphate (PI(3)P) in the ER, prior to and independent of protease action. Secretome HT signals, including those of major virulence determinants, bind PI(3)P with nanomolar affinity and amino acid specificities displayed by HT-mediated export. PI(3)P-enriched regions are detected within the parasite's ER and colocalize with endogenous HT signal on ER precursors, which also display high-affinity binding to PI(3)P. A related pathogenic oomycete's HT signal export is dependent on PI(3)P binding, without cleavage by plasmepsin V. Thus, PI(3)P in the ER functions in mechanisms of secretion and pathogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
References
-
- Crary JL, Haldar K. Brefeldin A inhibits protein secretion and parasite maturation in the ring stage of Plasmodium falciparum. Mol Biochem Parasitol. 1992;53:185–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

