Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N'-dimethyltryptamine forming enzyme, indole-N-methyl transferase
- PMID: 22265729
- PMCID: PMC3321351
- DOI: 10.1016/j.neuroscience.2011.12.040
Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N,N'-dimethyltryptamine forming enzyme, indole-N-methyl transferase
Abstract
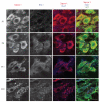
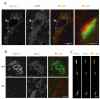
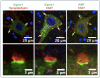
The function of the sigma-1 receptor (S1R) has been linked to modulating the activities of ion channels and G-protein-coupled receptors (GPCR). In the CNS, the S1R is expressed ubiquitously but is enriched in mouse motoneurons (MN), where it is localized to subsurface cisternae of cholinergic postsynaptic densities, also known as C-terminals. We found that S1R is enriched in mouse spinal MN at late stages of embryonic development when it is first visualized in the endoplasmic reticulum. S1Rs appear to concentrate at C-terminals of mouse MN only on the second week of postnatal development. We found that indole-N-methyl transferase (INMT), an enzyme that converts tryptamine into the sigma-1 ligand dimethyltryptamine (DMT), is also localized to postsynaptic sites of C-terminals in close proximity to the S1R. This close association of INMT and S1Rs suggest that DMT is synthesized locally to effectively activate S1R in MN.
Published by Elsevier Ltd.
Figures


References
-
- Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011 - PubMed
-
- Alvarez FJ, Pearson JC, Harrington D, Dewey D, Torbeck L, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on alpha-motoneurons in the lumbar spinal cord of adult cats. J Comp Neurol. 1998;393:69–83. - PubMed
-
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. - PubMed
-
- Aydar E, Palmer CP, Klyachko VA, Jackson MB. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

