RETRACTED: Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity
- PMID: 22266604
- PMCID: PMC4127883
- DOI: 10.1016/j.jhep.2011.12.019
RETRACTED: Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity
Retraction in
-
Retraction notice to "Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity": J Hepatol 56(2012)1070-1079.J Hepatol. 2020 Nov;73(5):1297. doi: 10.1016/j.jhep.2020.08.022. J Hepatol. 2020. PMID: 33070867 Free PMC article. No abstract available.
Expression of concern in
-
Expression of Concern.J Hepatol. 2018 Dec;69(6):1402. doi: 10.1016/j.jhep.2018.09.020. Epub 2018 Oct 16. J Hepatol. 2018. PMID: 30340775 No abstract available.
Abstract
Background & aims: Full length keratin-18 (FL-K18) and High Mobility Group Box-1 (HMGB1) represent circulating indicators of necrosis during acetaminophen (APAP) hepatotoxicity in vivo. In addition, the caspase-cleaved fragment of K18 (cK18) and hyper-acetylated HMGB1 represent serum indicators of apoptosis and immune cell activation, respectively. The study aim was to assess their mechanistic utility to establish the balance between apoptosis, necrosis, and immune cell activation throughout the time course of clinical APAP hepatotoxicity.
Methods: HMGB1 (total, acetylated) and K18 (apoptotic, necrotic) were identified and quantified by novel LC-MS/MS assays in APAP overdose patients (n=78).
Results: HMGB1 (total; 15.4±1.9ng/ml, p<0.01, acetylated; 5.4±2.6ng/ml, p<0.001), cK18 (5649.8±721.0U/L, p<0.01), and FL-K18 (54770.2±6717.0U/L, p<0.005) were elevated in the sera of APAP overdose patients with liver injury compared to overdose patients without liver injury and healthy volunteers. HMGB1 and FL-K18 correlated with alanine aminotransferase (ALT) activity (R(2)=0.60 and 0.58, respectively, p<0.0001) and prothrombin time (R(2)=0.62 and 0.71, respectively, p<0.0001). Increased total and acetylated HMGB1 and FL-K18 were associated with worse prognosis (King's College Criteria) or patients that died/required liver transplant compared to spontaneous survivors (all p<0.05-0.001), a finding not reflected by ALT and supported by ROC analysis. Acetylated HMGB1 was a better predictor of outcome than the other markers of cell death.
Conclusions: K18 and HMGB1 represent blood-based tools to investigate the cell death balance clinical APAP hepatotoxicity. Activation of the immune response was seen later in the time course as shown by the distinct profile of acetylated HMGB1 and was associated with worse outcome.
Copyright © 2012 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors wish to report no conflict of interest.
Figures
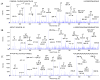


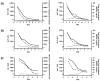


Comment in
-
Mechanistic biomarkers in acute liver injury: are we there yet?J Hepatol. 2012 May;56(5):1003-1005. doi: 10.1016/j.jhep.2012.01.017. Epub 2012 Feb 7. J Hepatol. 2012. PMID: 22322232 No abstract available.
References
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–954. - PubMed
-
- Starkey Lewis P, Dear J, Platt V, Simpson K, Craig D, Antoine D, et al. Circulating microRNAs as potential markers of human drug induced liver injury. Hepatology. 2011;54:1767–1776. - PubMed
-
- Antoine DJ, Williams DP, Kipar A, Jenkins RE, Sathish JG, Regan SL, et al. High Mobility Group Box-1 protein and Keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicological Sciences. 2009;112(2):521–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

