Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors
- PMID: 22266723
- PMCID: PMC3690506
- DOI: 10.1038/aps.2011.170
Structure and mechanism for recognition of peptide hormones by Class B G-protein-coupled receptors
Abstract
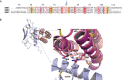
Class B G-protein-coupled receptors (GPCRs) are receptors for peptide hormones that include glucagon, parathyroid hormone, and calcitonin. These receptors are involved in a wide spectrum of physiological activities, from metabolic regulation and stress control to development and maintenance of the skeletal system. As such, they are important drug targets for the treatment of diabetes, osteoporosis, and stress related disorders. Class B GPCRs are organized into two modular domains: an extracellular domain (ECD) and a helical bundle that contains seven transmembrane helices (TM domain). The ECD is responsible for the high affinity and specificity of hormone binding, and the TM domain is required for receptor activation and signal coupling to downstream G-proteins. Although the structure of the full-length receptor remains unknown, the ECD structures have been well characterized for a number of Class B GPCRs, revealing a common fold for ligand recognition. This review summarizes the general structural principles that guide hormone binding by Class B ECDs and their implications in the design of peptide hormone analogs for therapeutic purposes.
Figures


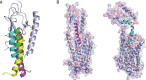
References
-
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. - PubMed
-
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–88. - PubMed
-
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

