Synergistic cytotoxic effect of sulindac and pyrrolidine dithiocarbamate against ovarian cancer cells
- PMID: 22266802
- PMCID: PMC3583429
- DOI: 10.3892/or.2012.1639
Synergistic cytotoxic effect of sulindac and pyrrolidine dithiocarbamate against ovarian cancer cells
Abstract
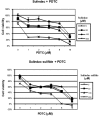
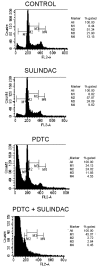
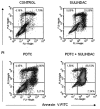
Sulindac, a non-steroidal anti-inflammatory drug, suppresses carcinogenesis and inhibits growth of tumor cells. Pyrrolidine dithiocarbamate (PDTC), a potent NF-κB inhibitor, has been also identified as a potential anti-neoplastic agent. We hypothesized that combination of sulindac and PDTC could result in augmentation of cytotoxicity against ovarian cancer cells. The effect of sulindac and PDTC was examined on several ovarian cancer lines. Tumor cell viability was assessed using the MTT assay. Annexin-V/PI staining was used to detect apoptosis, cell cycle distribution was analyzed in FACS, and expression of cellular proteins was detected by western blotting. Incubation of OVA-14, OVP-10 and CAOV-1 ovarian cancer cells with sulindac and PDTC resulted in significantly greater inhibition of cell viability compared to either compound alone. In a model of OVA-14 cells it was evident that this effect was not related to the expression of COX enzymes since both active (sulindac sulfide) and inactive (sulindac) in vitro compounds affected the growth of tumor cells to a similar extent and synergized in cytotoxicity with PDTC. Combination of sulindac and PDTC lead to G0 arrest and massive apoptosis in co-treated cultures. Western blotting analysis argued for induction of the mitochondrial apoptotic pathway. These data demonstrate the synergistic cytotoxic effect of sulindac and PDTC on ovarian cancer cells through apoptosis and cell cycle arrest and prompt to test the efficacy of this combination in animal models.
Figures


References
-
- Pautier P, Joly F, Kerbrat P, Bougnoux P, Fumoleau P, Petit T, Rixe O, Ringeisen F, Tisseron Carrasco A, Lhomme C. Phase II study of gefitinib in combination with paclitaxel (P) and carboplatin (C) as second-line therapy for ovarian, tubal or peritoneal adenocarcinoma (1839IL/0074) Gynecol Oncol. 2010;116:157–162. - PubMed
-
- Pectasides D, Pectasides E, Papaxoinis G, Psyrri A, Pliarchopoulou K, Koumarianou A, Macheras A, Athanasas G, Xiros N, Economopoulos T. Carboplatin/gemcitabine alternating with carboplatin/pegylated liposomal doxorubicin and carboplatin/cyclophosphamide in platinum-refractory/resistant paclitaxel-pretreated ovarian carcinoma. Gynecol Oncol. 2010;118:52–57. - PubMed
-
- Ledermann JA, Raja FA. Targeted trials in ovarian cancer. Gynecol Oncol. 2010;119:151–156. - PubMed
-
- Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

