Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells
- PMID: 22266857
- PMCID: PMC3337948
- DOI: 10.1038/onc.2011.633
Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells
Abstract
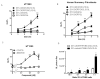
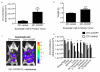
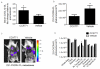
Chemokine CXCL12 and receptor CXCR4 control multiple steps in primary tumor growth and metastasis in breast cancer and more than 20 other human malignancies. Mechanisms that regulate availability of CXCL12 in tumor microenvironments will substantially impact cancer progression and ongoing efforts to target the CXCL12-CXCR4 pathway for cancer chemotherapy. We used dual luciferase imaging to investigate CXCR7-dependent scavenging of CXCL12 in breast tumors in vivo and quantify effects of CXCR7 on tumor growth and metastasis of a separate population of CXCR4+ breast cancer cells. In a mouse xenograft model of human breast cancer, in vivo imaging showed that malignant cells expressing CXCR7 reduced bioluminescent CXCL12 secreted in the primary tumor microenvironment. Capitalizing on sensitive detection of bioluminescent CXCL12, we also demonstrated that CXCR7+ cells reduced amounts of chemokine released from orthotopic tumors into the circulation. Immunofluorescence staining of human primary breast cancers showed expression of CXCR4 and CXCR7 on malignant cells in ≈30% of cases. In most cases, CXCR4 and CXCR7 predominantly were expressed on separate populations of malignant cells in a tumor. We modeled these cases of human breast cancer by co-implanting tumor xenografts with CXCR4+ breast cancer cells, human mammary fibroblasts secreting CXCL12, and CXCR7+ or control breast cancer cells. Bioluminescence imaging showed that CXCR7+ breast cancer cells enhanced proliferation of CXCR4+ breast cancer cells in orthotopic tumors and spontaneous metastases. Treatment with a small-molecule inhibitor of CXCR7 chemokine limited the growth of CXCR4+ breast cancer cells in tumors that also contained malignant CXCR7+ cells. These studies establish a new in vivo imaging method to quantify chemokine scavenging by CXCR7 in the tumor microenvironment and identify that CXCR7+ cells promote growth and metastasis of CXCR4+ breast cancer cells.
Figures



References
-
- Smith M, Luker K, Garbow J, Prior J, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. - PubMed
-
- Orimo A, Gupta P, Sgroi D, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. - PubMed
-
- Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. - PubMed
-
- Lee B, Lee T, Avraham S, Avraham H. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Mol Cancer Res. 2004;2:327–338. - PubMed
-
- Muller A, Homey B, Soto H, Ge N, Catron D, Buchanon M, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

