Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma
- PMID: 22266870
- PMCID: PMC3730824
- DOI: 10.1038/onc.2011.647
Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma
Erratum in
- Oncogene. 2012 Nov 15;31(46):4888. Vigny, M [added]; Mazot, P [added]
Abstract
Anaplastic lymphoma kinase (ALK) is a receptor tyrosine kinase aberrantly expressed in neuroblastoma, a devastating pediatric cancer of the sympathetic nervous system. Germline and somatically acquired ALK aberrations induce increased autophosphorylation, constitutive ALK activation and increased downstream signaling. Thus, ALK is a tractable therapeutic target in neuroblastoma, likely to be susceptible to both small-molecule tyrosine kinase inhibitors and therapeutic antibodies-as has been shown for other receptor tyrosine kinases in malignancies such as breast and lung cancer. Small-molecule inhibitors of ALK are currently being studied in the clinic, but common ALK mutations in neuroblastoma appear to show de novo insensitivity, arguing that complementary therapeutic approaches must be developed. We therefore hypothesized that antibody targeting of ALK may be a relevant strategy for the majority of neuroblastoma patients likely to have ALK-positive tumors. We show here that an antagonistic ALK antibody inhibits cell growth and induces in vitro antibody-dependent cellular cytotoxicity of human neuroblastoma-derived cell lines. Cytotoxicity was induced in cell lines harboring either wild type or mutated forms of ALK. Treatment of neuroblastoma cells with the dual Met/ALK inhibitor crizotinib sensitized cells to antibody-induced growth inhibition by promoting cell surface accumulation of ALK and thus increasing the accessibility of antigen for antibody binding. These data support the concept of ALK-targeted immunotherapy as a highly promising therapeutic strategy for neuroblastomas with mutated or wild-type ALK.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
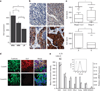

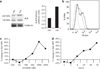

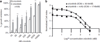

References
-
- Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. - PubMed
-
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. - PubMed
-
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. - PubMed
-
- Griffin CA, Hawkins AL, Dvorak C, Henkle C, Ellingham T, Perlman EJ. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59:2776–2780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

