Diversity oriented syntheses of conventional heterocycles by smart multi component reactions (MCRs) of the last decade
- PMID: 22267194
- PMCID: PMC6268852
- DOI: 10.3390/molecules17011074
Diversity oriented syntheses of conventional heterocycles by smart multi component reactions (MCRs) of the last decade
Abstract
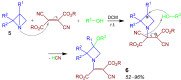
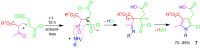
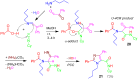
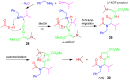
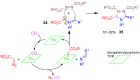
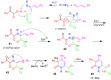
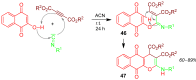
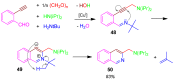
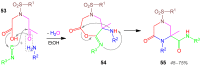
A collection of smart multicomponent reactions (MCRs) with continuative post condensation cyclizations (PCCs) is presented to construct conventional three- to seven-membered heterocyclic compounds in diversity oriented syntheses (DOS). These will provide a high degree of applying economical and ecological advantages as well as of practicability. Water, ionic liquids, and solvent-less syntheses as well as use of various forms of energy as microwave and ultrasonic irradiation are examined and discussed.
Figures

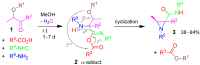
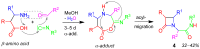

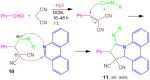
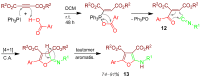
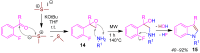
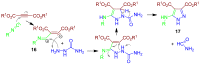


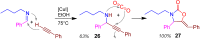




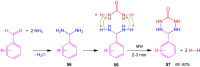
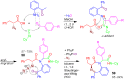
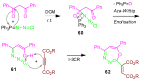
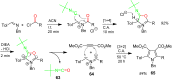
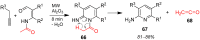

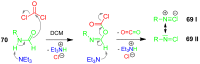
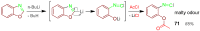
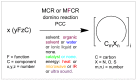
References
-
- Zhu J., Bienayme H., editors. Multicomponent Reactions. Wiley-VCH; Weinheim, Germany: 2005.
-
- Eckert H. From multicomponent-reactions (MCRs) towards multi-function-component-reactions (MFCRs) Heterocycles. 2007;73:149–158. doi: 10.3987/REV-07-SR(U)4. - DOI
-
- Katritzky A.R., Ramsden C.A., Scriven E.F.V., Taylor R.J.K., editors. Comprehensive Heterocyclic Chemistry III. Elsevier; New York, NY, USA: 2008.
-
- Pozharskii A.F., Soldatenkov A.T., Katritzky A.R. Heterocycles in Life and Society. 2nd. Wiley & Sons; New York, NY, USA: 2011.
-
- Hemming K. Heterocyclic chemistry. Annu. Rep. Prog. Chem. B. 2011;107:118–137. doi: 10.1039/c1oc90016a. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

