Extracellular matrix dynamics and fetal membrane rupture
- PMID: 22267536
- PMCID: PMC3826277
- DOI: 10.1177/1933719111424454
Extracellular matrix dynamics and fetal membrane rupture
Abstract
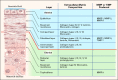
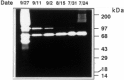
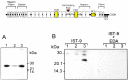
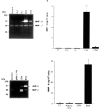
The extracellular matrix (ECM) plays an important role in determining cell and organ function: (1) it is an organizing substrate that provides tissue tensile strength; (2) it anchors cells and influences cell morphology and function via interaction with cell surface receptors; and (3) it is a reservoir for growth factors. Alterations in the content and the composition of the ECM determine its physical and biological properties, including strength and susceptibility to degradation. The ECM components themselves also harbor cryptic matrikines, which when exposed by conformational change or proteolysis have potent effects on cell function, including stimulating the production of cytokines and matrix metalloproteinases (MMPs). Collectively, these properties of the ECM reflect a dynamic tissue component that influences both tissue form and function. This review illustrates how defects in ECM synthesis and metabolism and the physiological process of ECM turnover contribute to changes in the fetal membranes that precede normal parturition and contribute to the pathological events leading to preterm premature rupture of membranes (PPROM).
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

