Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial
- PMID: 22267714
- PMCID: PMC3297650
- DOI: 10.1093/cid/cir968
Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial
Abstract
Background: The study goal was to determine the effect of vitamin D (VITD) supplementation on tubular reabsorption of phosphate (TRP), parathyroid hormone (PTH), bone alkaline phosphatase (BAP), and C-telopeptide (CTX) in youth infected with human immunodeficiency virus (HIV) receiving and not receiving combination antiretroviral therapy (cART) containing tenofovir disoproxil fumarate (TDF).
Methods: This randomized, double-blind, placebo-controlled multicenter trial enrolled HIV-infected youth 18-25 years based on stable treatment with cART containing TDF (n = 118) or no TDF (noTDF; n = 85), and randomized within those groups to vitamin D3, 50 000 IU (n = 102) or placebo (n = 101), administered at 0, 4, and 8 weeks. Outcomes included change in TRP, PTH, BAP, and CTX from baseline to week 12 by TDF/noTDF; and VITD/placebo.
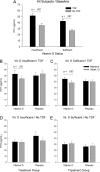
Results: At baseline, VITD and placebo groups were similar except those on TDF had lower TRP and higher PTH and CTX. At week 12, 95% in the VITD group had sufficient serum 25-hydroxy vitamin D (25-OHD; ≥20 ng/mL), increased from 48% at baseline, without change in placebo (P < .001). PTH decreased in the TDF group receiving VITD (P = .031) but not in the noTDF group receiving VITD, or either placebo group. The decrease in PTH with VITD in those on TDF occurred with insufficient and sufficient baseline 25-OHD (mean PTH change, -7.9 and -6.2 pg/mL; P = .031 and .053, respectively).
Conclusions: In youth on TDF, vitamin D3 supplementation decreased PTH, regardless of baseline 25-OHD concentration.
Clinical trials registration: NCT00490412.
Figures
References
-
- Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51:496–505. - PubMed
-
- Judd A, Boyd KL, Stohr W, et al. Effect of tenofovir disoproxil fumarate on risk of renal abnormality in HIV-1-infected children on antiretroviral therapy: a nested case-control study. AIDS. 2010;24:525–34. - PubMed
-
- Kinai E, Hanabusa H. Renal tubular toxicity associated with tenofovir assessed using urine-beta 2 microglobulin, percentage of tubular reabsorption of phosphate and alkaline phosphatase levels. AIDS. 2005;19:2031–3. - PubMed
-
- Kinai E, Hanabusa H, Kinai E, Hanabusa H. Progressive renal tubular dysfunction associated with long-term use of tenofovir DF. AIDS Res Hum Retroviruses. 2009;25:387–94. - PubMed
-
- Rodriguez-Novoa S, Labarga P, Soriano V, et al. Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108–16. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1-RR02517/RR/NCRR NIH HHS/United States
- M01-RR00188/RR/NCRR NIH HHS/United States
- U01 HD040497/HD/NICHD NIH HHS/United States
- M01 RR000188/RR/NCRR NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- M01 RR010710/RR/NCRR NIH HHS/United States
- U01 HD 040533/HD/NICHD NIH HHS/United States
- UL1-RR025014/RR/NCRR NIH HHS/United States
- U01 HD040533/HD/NICHD NIH HHS/United States
- U01 HD 040474/HD/NICHD NIH HHS/United States
- U01 HD040474/HD/NICHD NIH HHS/United States
- M01-RR10710/RR/NCRR NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- U01 A1068632/PHS HHS/United States
- UL1-RR-024134/RR/NCRR NIH HHS/United States
- M01RR020359/RR/NCRR NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- M01 RR020359/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

