A cell culture-derived influenza vaccine provides consistent protection against infection and reduces the duration and severity of disease in infected individuals
- PMID: 22267715
- PMCID: PMC3297649
- DOI: 10.1093/cid/cir959
A cell culture-derived influenza vaccine provides consistent protection against infection and reduces the duration and severity of disease in infected individuals
Abstract
Background: Current knowledge of the consistency of protection induced by seasonal influenza vaccines over the duration of a full influenza season is limited, and little is known about the clinical course of disease in individuals who become infected despite vaccination.
Methods: Data from a randomized double-blind placebo-controlled clinical trial undertaken in healthy young adults in the 2008-2009 influenza season were used to investigate the weekly cumulative efficacy of a Vero cell culture-derived influenza vaccine. In addition, the duration and severity of disease in vaccine and placebo recipients with cell culture-confirmed influenza infection were compared.
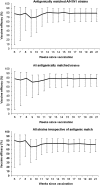
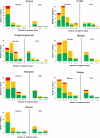
Results: Vaccine efficacy against matching strains was consistently high (73%-82%) throughout the study, including the entire period of the influenza season during which influenza activity was above the epidemic threshold. Vaccine efficacy was also consistent (68%-83%) when calculated for all strains, irrespective of antigenic match. Vaccination also ameliorated disease symptoms when infection was not prevented. Bivariate analysis of duration and severity showed a significant amelioration of myalgia (P = .003), headache (P = .025), and fatigue (P = .013) in infected vaccinated subjects compared with placebo. Cough (P = .143) and oropharyngeal pain (P = .083) were also reduced in infected vaccinated subjects.
Conclusions: A Vero cell culture-derived influenza vaccine provides consistently high levels of protection against cell culture-confirmed infection by seasonal influenza virus and significantly reduces the duration and severity of disease in those individuals in which infection is not prevented.
Clinical trials registration: ClinicalTrials.gov NCT00566345.
Figures

References
-
- Barrett PN, Berezuk G, Fritsch S, et al. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2011;377:751–9. - PubMed
-
- Frey S, Vesikari T, Szymczakiewicz-Multanowska A, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. - PubMed
-
- Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. - PubMed
-
- Beran J, Vesikari T, Wertzova V, et al. Efficacy of inactivated split-virus influenza vaccine against culture-confirmed influenza in healthy adults: a prospective, randomized, placebo-controlled trial. J Infect Dis. 2009;200:1861–9. - PubMed

