Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton
- PMID: 22267745
- PMCID: PMC3318751
- DOI: 10.1074/jbc.M111.306522
Pannexin1 drives multicellular aggregate compaction via a signaling cascade that remodels the actin cytoskeleton
Abstract
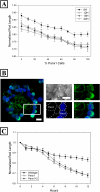
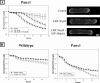
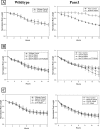
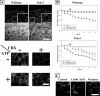
Pannexin 1 (Panx1) is a novel gap junction protein shown to have tumor-suppressive properties. To model its in vivo role in the intratumor biomechanical environment, we investigated whether Panx1 channels modulate the dynamic assembly of multicellular C6 glioma aggregates. Treatment with carbenoxolone and probenecid, which directly and specifically block Panx1 channels, respectively, showed that Panx1 is involved in accelerating aggregate assembly. Experiments further showed that exogenous ATP can reverse the inhibitive effects of carbenoxolone and that aggregate compaction is sensitive to the purinergic antagonist suramin. With a close examination of the F-actin microfilament network, these findings show that Panx1 channels act as conduits for ATP release that stimulate the P(2)X(7) purinergic receptor pathway, in turn up-regulating actomyosin function. Using a unique three-dimensional scaffold-free method to quantify multicellular interactions, this study shows that Panx1 is intimately involved in regulating intercellular biomechanical interactions pivotal in the progression of cancer.
Figures

References
-
- Baranova A., Ivanov D., Petrash N., Pestova A., Skoblov M., Kelmanson I., Shagin D., Nazarenko S., Geraymovych E., Litvin O., Tiunova A., Born T. L., Usman N., Staroverov D., Lukyanov S., Panchin Y. (2004) The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83, 706–716 - PubMed
-
- Penuela S., Bhalla R., Gong X. Q., Cowan K. N., Celetti S. J., Cowan B. J., Bai D., Shao Q., Laird D. W. (2007) Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J. Cell Sci. 120, 3772–3783 - PubMed
-
- Perkins G. A., Goodenough D. A., Sosinsky G. E. (1998) Formation of the gap junction intercellular channel requires a 30 degree rotation for interdigitating two apposing connexons. J. Mol. Biol. 277, 171–177 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

