Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method
- PMID: 22267927
- PMCID: PMC3260036
- DOI: 10.2147/IJN.S27157
Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method
Abstract
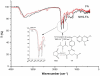
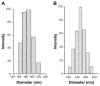
Oligomycin-A (Oli-A), an anticancer drug, was loaded to the folate (FA)-conjugated chitosan as a tumor-targeted drug delivery system for the purpose of overcoming the nonspecific targeting characteristics and the hydrophobicity of the compound. The two-level factorial design (2-LFD) was applied to modeling the preparation process, which was composed of five independent variables, namely FA-conjugated chitosan (FA-CS) concentration, Oli-A concentration, sodium tripolyphosphate (TPP) concentration, the mass ratio of FA-CS to TPP, and crosslinking time. The mean particle size (MPS) and the drug loading rate (DLR) of the resulting Oli-loaded FA-CS nanoparticles (FA-Oli-CSNPs) were used as response variables. The interactive effects of the five independent variables on the response variables were studied. The characteristics of the nanoparticles, such as amount of FA conjugation, drug entrapment rate (DER), DLR, surface morphology, and release kinetics properties in vitro were investigated. The FA-Oli-CSNPs with MPS of 182.6 nm, DER of 17.3%, DLR of 58.5%, and zeta potential (ZP) of 24.6 mV were obtained under optimum conditions. The amount of FA conjugation was 45.9 mg/g chitosan. The FA-Oli-CSNPs showed sustained-release characteristics for 576 hours in vitro. The results indicated that FA-Oli-CSNPs obtained as a targeted drug delivery system could be effective in the therapy of leukemia in the future.
Keywords: chitosan; folate; nanoparticles; oligomycin-A; targeted drug delivery system; two-level factorial design.
Figures


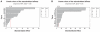






References
-
- Kihara T, Kusakabe H, Nakamura G, Sakurai T, Isono K. Cytovaricin, a novel antibiotic. J Antibiot (Tokyo) 1981;34:1073–1074. - PubMed
-
- Kobayashi K, Nishino C, Ohya J, et al. Oligomycin E, a new antitumor antibiotic produced by Streptomyces sp. MCI-2225. J Antibiot (Tokyo) 1987;40:1053–1057. - PubMed
-
- Tanaka Y, Omura S. Agroactive compounds of microbial origin. Annu Rev Microbiol. 1993;47:57–87. - PubMed
-
- Enomoto Y, Shiomi K, Matsumoto A, et al. Isolation of a new antibiotic oligomycin G produced by Streptomyces sp. WK-6150. J Antibiot (Tokyo) 2001;54:308–313. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

