Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus
- PMID: 22267935
- PMCID: PMC3258012
- DOI: 10.2147/IJGM.S26889
Pharmacokinetics and pharmacodynamics of insulin analogs in special populations with type 2 diabetes mellitus
Abstract
Introduction: The goal of insulin therapy in patients with either type 1 diabetes mellitus (T1DM) or type 2 diabetes mellitus (T2DM) is to match as closely as possible normal physiologic insulin secretion to control fasting and postprandial plasma glucose. Modifications of the insulin molecule have resulted in two long-acting insulin analogs (glargine and detemir) and three rapid-acting insulins (aspart, lispro, and glulisine) with improved pharmacokinetic/pharmacodynamic (PK/PD) profiles. These agents can be used together in basal-bolus therapy to more closely mimic physiologic insulin secretion patterns.
Methods: This study reviews effects of the multiple demographic and clinical parameters in the insulin analogs glargine, detemir, lispro, aspart, and glulisine in patients with T2DM. A search was conducted on PubMed for each major topic considered (effects of injection site, age, race/ethnicity, obesity, renal or hepatic dysfunction, pregnancy, exercise, drug interactions) using the topic words and name of each type of insulin analog. Information was also obtained from the prescribing information for each insulin analog.
Results: The PK/PD profiles for insulin analogs may be influenced by many variables including age, weight, and hepatic and renal function. However, these variables do not have equivalent effects on all long-acting or rapid-acting insulin analogs.
Conclusion: Rapid-acting and long-acting insulin analogs represent major advances in treatment for patients with T2DM who require insulin therapy. However, there are potentially important PK and PD differences between the two long-acting agents and among the three rapid-acting insulin analogs, which should be considered when designing treatment regimens for special patient groups.
Keywords: insulin analogs; pharmacodynamics; pharmacokinetics; type 2 diabetes mellitus.
Figures
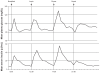
Similar articles
-
Insulin analogs in the treatment of type II diabetes and future perspectives.Dis Mon. 2023 Mar;69(3):101417. doi: 10.1016/j.disamonth.2022.101417. Epub 2022 Apr 27. Dis Mon. 2023. PMID: 35487767 Review.
-
Understanding how pharmacokinetic and pharmacodynamic differences of basal analog insulins influence clinical practice.Curr Med Res Opin. 2017 Oct;33(10):1821-1831. doi: 10.1080/03007995.2017.1335192. Epub 2017 Jun 23. Curr Med Res Opin. 2017. PMID: 28537449 Review.
-
Insulin analogs: Glimpse on contemporary facts and future prospective.Life Sci. 2019 Feb 15;219:90-99. doi: 10.1016/j.lfs.2019.01.011. Epub 2019 Jan 10. Life Sci. 2019. PMID: 30639280 Review.
-
Beyond the era of NPH insulin--long-acting insulin analogs: chemistry, comparative pharmacology, and clinical application.Diabetes Technol Ther. 2008 Oct;10(5):333-49. doi: 10.1089/dia.2008.0023. Diabetes Technol Ther. 2008. PMID: 18715209 Review.
-
Superiority of insulin analogues versus human insulin in the treatment of diabetes mellitus.Arch Physiol Biochem. 2008 Feb;114(1):3-10. doi: 10.1080/13813450801900777. Arch Physiol Biochem. 2008. PMID: 18465353 Review.
Cited by
-
Polymer conjugation of proteins as a synthetic post-translational modification to impact their stability and activity.Polym Chem. 2019 Jan 28;10(4):434-454. doi: 10.1039/C8PY01399C. Epub 2018 Dec 7. Polym Chem. 2019. PMID: 31249635 Free PMC article.
-
Effect of insulin analog initiation therapy on LDL/HDL subfraction profile and HDL associated enzymes in type 2 diabetic patients.Lipids Health Dis. 2013 Apr 24;12:54. doi: 10.1186/1476-511X-12-54. Lipids Health Dis. 2013. PMID: 23617853 Free PMC article.
-
A review of the pharmacological properties of insulin degludec and their clinical relevance.Clin Pharmacokinet. 2014 Sep;53(9):787-800. doi: 10.1007/s40262-014-0165-y. Clin Pharmacokinet. 2014. PMID: 25179915 Free PMC article. Review.
-
Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems.Nat Rev Drug Discov. 2023 May;22(5):387-409. doi: 10.1038/s41573-023-00670-0. Epub 2023 Mar 27. Nat Rev Drug Discov. 2023. PMID: 36973491 Free PMC article. Review.
-
Glucose-lowering effect of insulin degludec is independent of subcutaneous injection region.Clin Drug Investig. 2014 Sep;34(9):673-9. doi: 10.1007/s40261-014-0218-x. Clin Drug Investig. 2014. PMID: 25124362 Free PMC article. Clinical Trial.
References
-
- Bogardus C, Tataranni PA. Reduced early insulin secretion in the etiology of type 2 diabetes mellitus in Pima Indians. Diabetes. 2002;51(Suppl 1):S262–S264. - PubMed
-
- King AB, Armstrong DU. Basal bolus dosing: a clinical experience. Curr Diabetes Rev. 2005;1:215–220. - PubMed
-
- Gerich JE. Novel insulins: expanding options in diabetes management. Am J Med. 2002;113:308–316. - PubMed
-
- Levemir® (insulin detemir [rDNA origin] injection) [prescribing information] Princeton, NJ: Novo Nordisk Inc; 2010.
-
- Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. - PubMed

