Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr-/-:hApoB+/+ mice and improves cerebral blood flow
- PMID: 22268108
- PMCID: PMC3695886
- DOI: 10.1152/ajpheart.01044.2011
Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr-/-:hApoB+/+ mice and improves cerebral blood flow
Abstract
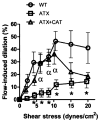
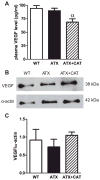
Endothelial dysfunction and oxidative stress contribute to the atherosclerotic process that includes stiffening of large peripheral arteries. In contrast, our laboratory previously reported a paradoxical increase in cerebrovascular compliance in LDLr(-/-):hApoB(+/+) atherosclerotic (ATX) mice (7). We hypothesized that prevention of cerebral artery endothelial dysfunction with a chronic dietary antioxidant intake would normalize the changes in cerebral artery wall structure and biomechanics and prevent the decline in basal cerebral blood flow associated with atherosclerosis. Three-month-old ATX mice were treated, or not, for 3 mo with the polyphenol (+)-catechin (CAT; 30 mg·kg(-1)·day(-1)) and compared with wild-type controls. In isolated, pressurized cerebral arteries from ATX mice, CAT prevented endothelial dysfunction (deterioration of endothelium-dependent, flow-mediated dilations; P < 0.05), the inward hypertrophic structural remodeling (increase in the wall-to-lumen ratio; P < 0.05), and the rise in cerebrovascular compliance (rightward shift of the stress-strain curve measured in passive conditions, reflecting mechanical properties of the arterial wall; P < 0.05). Doppler optical coherence tomography imaging in vivo confirmed these findings, showing that cerebral compliance was higher in ATX mice and normalized by CAT (P < 0.05). CAT also prevented basal cerebral hypoperfusion in ATX mice (P < 0.05). Active remodeling of the cerebrovascular wall in ATX mice was further suggested by the increase (P < 0.05) in pro-metalloproteinase-9 activity, which was normalized by CAT. We conclude that, by preserving the endothelial function, a chronic treatment with CAT prevents the deleterious effect of severe dyslipidemia on cerebral artery wall structure and biomechanical properties, contributing to preserving resting cerebral blood flow.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the author(s).
Figures




Similar articles
-
Heart rate-associated mechanical stress impairs carotid but not cerebral artery compliance in dyslipidemic atherosclerotic mice.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H2081-92. doi: 10.1152/ajpheart.00706.2011. Epub 2011 Sep 16. Am J Physiol Heart Circ Physiol. 2011. PMID: 21926346 Free PMC article.
-
Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice.Am J Physiol Heart Circ Physiol. 2011 Mar;300(3):H1032-43. doi: 10.1152/ajpheart.00410.2010. Epub 2010 Dec 24. Am J Physiol Heart Circ Physiol. 2011. PMID: 21186270 Free PMC article.
-
Late chronic catechin antioxidant treatment is deleterious to the endothelial function in aging mice with established atherosclerosis.Am J Physiol Heart Circ Physiol. 2010 Jun;298(6):H2062-70. doi: 10.1152/ajpheart.00532.2009. Epub 2010 Apr 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20382853 Free PMC article.
-
Up-regulation of thromboxane A₂ impairs cerebrovascular eNOS function in aging atherosclerotic mice.Pflugers Arch. 2011 Sep;462(3):371-83. doi: 10.1007/s00424-011-0973-y. Epub 2011 May 27. Pflugers Arch. 2011. PMID: 21617900 Free PMC article.
-
Endothelial TRPV4 channels mediate dilation of cerebral arteries: impairment and recovery in cerebrovascular pathologies related to Alzheimer's disease.Br J Pharmacol. 2013 Oct;170(3):661-70. doi: 10.1111/bph.12315. Br J Pharmacol. 2013. PMID: 23889563 Free PMC article.
Cited by
-
Targeting angiopoietin like-2 positive senescent cells improves cognitive impairment in adult male but not female atherosclerotic LDLr-/-;hApoB100+/+ mice.Geroscience. 2025 Jun 30. doi: 10.1007/s11357-025-01763-x. Online ahead of print. Geroscience. 2025. PMID: 40587054
-
The therapeutic potential of matcha tea: A critical review on human and animal studies.Curr Res Food Sci. 2022 Nov 23;6:100396. doi: 10.1016/j.crfs.2022.11.015. eCollection 2023. Curr Res Food Sci. 2022. PMID: 36582446 Free PMC article. Review.
-
Hydrophilic extract from Posidonia oceanica inhibits activity and expression of gelatinases and prevents HT1080 human fibrosarcoma cell line invasion.Cell Adh Migr. 2015;9(6):422-31. doi: 10.1080/19336918.2015.1008330. Epub 2015 Jul 15. Cell Adh Migr. 2015. PMID: 26176658 Free PMC article.
-
Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline.Am J Physiol Heart Circ Physiol. 2018 Jun 1;314(6):H1214-H1224. doi: 10.1152/ajpheart.00637.2017. Epub 2018 Feb 16. Am J Physiol Heart Circ Physiol. 2018. PMID: 29451817 Free PMC article. Review.
-
Epigenetic Regulatory Effect of Exercise on Glutathione Peroxidase 1 Expression in the Skeletal Muscle of Severely Dyslipidemic Mice.PLoS One. 2016 Mar 24;11(3):e0151526. doi: 10.1371/journal.pone.0151526. eCollection 2016. PLoS One. 2016. PMID: 27010651 Free PMC article.
References
-
- Albaladejo P, Carusi A, Apartian A, Lacolley P, Safar ME, Benetos A. Effect of chronic heart rate reduction with ivabradine on carotid and aortic structure and function in normotensive and hypertensive rats. J Vasc Res. 2003;40:320–328. - PubMed
-
- Auclair S, Milenkovic D, Besson C, Chauvet S, Gueux E, Morand C, Mazur A, Scalbert A. Catechin reduces atherosclerotic lesion development in apo E-deficient mice: a transcriptomic study. Atherosclerosis. 2009;204:e21–e27. - PubMed
-
- Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

