Coenzyme Q(1) as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung
- PMID: 22268123
- PMCID: PMC3362155
- DOI: 10.1152/ajplung.00251.2011
Coenzyme Q(1) as a probe for mitochondrial complex I activity in the intact perfused hyperoxia-exposed wild-type and Nqo1-null mouse lung
Abstract
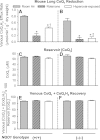
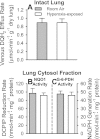
Previous studies showed that coenzyme Q(1) (CoQ(1)) reduction on passage through the rat pulmonary circulation was catalyzed by NAD(P)H:quinone oxidoreductase 1 (NQO1) and mitochondrial complex I, but that NQO1 genotype was not a factor in CoQ(1) reduction on passage through the mouse lung. The aim of the present study was to evaluate the complex I contribution to CoQ(1) reduction in the isolated perfused wild-type (NQO1(+/+)) and Nqo1-null (NQO1(-)/(-)) mouse lung. CoQ(1) reduction was measured as the steady-state pulmonary venous CoQ(1) hydroquinone (CoQ(1)H(2)) efflux rate during infusion of CoQ(1) into the pulmonary arterial inflow. CoQ(1)H(2) efflux rates during infusion of 50 μM CoQ(1) were not significantly different for NQO1(+/+) and NQO1(-/-) lungs (0.80 ± 0.03 and 0.68 ± 0.07 μmol·min(-1)·g lung dry wt(-1), respectively, P > 0.05). The mitochondrial complex I inhibitor rotenone depressed CoQ(1)H(2) efflux rates for both genotypes (0.19 ± 0.08 and 0.08 ± 0.04 μmol·min(-1)·g lung dry wt(-1) for NQO1(+/+) and NQO1(-/-), respectively, P < 0.05). Exposure of mice to 100% O(2) for 48 h also depressed CoQ(1)H(2) efflux rates in NQO1(+/+) and NQO1(-/-) lungs (0.43 ± 0.03 and 0.11 ± 0.04 μmol·min(-1)·g lung dry wt(-1), respectively, P < 0.05 by ANOVA). The impact of rotenone or hyperoxia on CoQ(1) redox metabolism could not be attributed to effects on lung wet-to-dry weight ratios, perfusion pressures, perfused surface areas, or total venous effluent CoQ(1) recoveries, the latter measured by spectrophotometry or mass spectrometry. Complex I activity in mitochondria-enriched lung fractions was depressed in hyperoxia-exposed lungs for both genotypes. This study provides new evidence for the potential utility of CoQ(1) as a nondestructive indicator of the impact of pharmacological or pathological exposures on complex I activity in the intact perfused mouse lung.
Figures




Similar articles
-
Genetic evidence for NAD(P)H:quinone oxidoreductase 1-catalyzed quinone reduction on passage through the mouse pulmonary circulation.Am J Physiol Lung Cell Mol Physiol. 2011 May;300(5):L773-80. doi: 10.1152/ajplung.00394.2010. Epub 2011 Feb 4. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21296895 Free PMC article.
-
Coenzyme Q1 redox metabolism during passage through the rat pulmonary circulation and the effect of hyperoxia.J Appl Physiol (1985). 2008 Oct;105(4):1114-26. doi: 10.1152/japplphysiol.00177.2008. Epub 2008 Aug 14. J Appl Physiol (1985). 2008. PMID: 18703762 Free PMC article.
-
Role of mitochondrial electron transport complex I in coenzyme Q1 reduction by intact pulmonary arterial endothelial cells and the effect of hyperoxia.Am J Physiol Lung Cell Mol Physiol. 2007 Sep;293(3):L809-19. doi: 10.1152/ajplung.00448.2006. Epub 2007 Jun 29. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17601793
-
Idebenone and neuroprotection: antioxidant, pro-oxidant, or electron carrier?J Bioenerg Biomembr. 2015 Apr;47(1-2):111-8. doi: 10.1007/s10863-014-9571-y. Epub 2014 Sep 28. J Bioenerg Biomembr. 2015. PMID: 25262284 Free PMC article. Review.
-
An updating of the biochemical function of coenzyme Q in mitochondria.Mol Aspects Med. 1994;15 Suppl:s29-36. doi: 10.1016/0098-2997(94)90010-8. Mol Aspects Med. 1994. PMID: 7752842 Review.
Cited by
-
Depleted energy charge and increased pulmonary endothelial permeability induced by mitochondrial complex I inhibition are mitigated by coenzyme Q1 in the isolated perfused rat lung.Free Radic Biol Med. 2013 Dec;65:1455-1463. doi: 10.1016/j.freeradbiomed.2013.07.040. Epub 2013 Aug 1. Free Radic Biol Med. 2013. PMID: 23912160 Free PMC article.
-
Mitochondrial dysfunction and pulmonary hypertension: cause, effect, or both.Am J Physiol Lung Cell Mol Physiol. 2018 May 1;314(5):L782-L796. doi: 10.1152/ajplung.00331.2017. Epub 2018 Jan 18. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29345195 Free PMC article. Review.
-
Redox regulation of ion channels in the pulmonary circulation.Antioxid Redox Signal. 2015 Feb 20;22(6):465-85. doi: 10.1089/ars.2014.5899. Epub 2014 Jun 30. Antioxid Redox Signal. 2015. PMID: 24702125 Free PMC article. Review.
References
-
- Allen CB, Guo XL, White CW. Changes in pulmonary expression of hexokinase and glucose transporter mRNAs in rats adapted to hyperoxia. Am J Physiol Lung Cell Mol Physiol 274: L320–L329, 1998 - PubMed
-
- Audi SH, Bongard RD, Dawson CA, Siegel D, Roerig DL, Merker MP. Duroquinone reduction during passage through the pulmonary circulation. Am J Physiol Lung Cell Mol Physiol 285: L1116–L1131, 2003 - PubMed
-
- Audi SH, Bongard RD, Okamoto Y, Merker MP, Roerig DL, Dawson CA. Pulmonary reduction of an intravascular redox polymer. Am J Physiol Lung Cell Mol Physiol 280: L1290–L1299, 2001 - PubMed
-
- Audi SH, Olson LE, Bongard RD, Roerig DL, Schulte ML, Dawson CA. Toluidine blue O and methylene blue as endothelial redox probes in the intact lung. Am J Physiol Heart Circ Physiol 278: H137–H150, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

