A large subgroup of mild-to-moderate asthma is persistently noneosinophilic
- PMID: 22268133
- PMCID: PMC3326288
- DOI: 10.1164/rccm.201109-1640OC
A large subgroup of mild-to-moderate asthma is persistently noneosinophilic
Abstract
Rationale: Airway eosinophilia is typical of asthma, and many controller treatments target eosinophilic disease. Asthma is clinically heterogeneous, however, and a subgroup of people with asthma do not have airway eosinophilia. The size of this subgroup is uncertain because prior studies have not examined repeated measures of sputum cytology to determine when people with asthma have intermittent versus persistent sputum eosinophila and when they are persistently noneosinophilic.
Objectives: To determine the prevalence and clinical characteristics of the noneosinophilic asthma phenotype.
Methods: We analyzed sputum cytology data from 995 subjects with asthma enrolled in clinical trials in the Asthma Clinical Research Network where they had undergone sputum induction and measures of sputum cytology, often repeatedly, and assessment of responses to standardized asthma treatments.
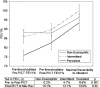
Measurements and main results: In cross-sectional analyses, sputum eosinophilia (≥2% eosinophils) was found in only 36% of subjects with asthma not taking an inhaled corticosteroid (ICS) and 17% of ICS-treated subjects with asthma; an absence of eosinophilia was noted frequently, even in subjects with asthma whose disease was suboptimally controlled. In repeated measures analyses of people with asthma not taking an ICS, 22% of subjects had sputum eosinophilia on every occasion (persistent eosinophilia); 31% had eosinophilia on at least one occasion (intermittent eosinophilia); and 47% had no eosinophilia on every occasion (persistently noneosinophilic). Two weeks of combined antiinflammatory therapy caused significant improvements in airflow obstruction in eosinophilic asthma, but not in persistently noneosinophilic asthma. In contrast, bronchodilator responses to albuterol were similar in eosinophilic and noneosinophilic asthma.
Conclusions: Approximately half of patients with mild-to-moderate asthma have persistently noneosinophilic disease, a disease phenotype that responds poorly to currently available antiinflammatory therapy.
Figures


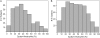


References
-
- Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002–2007. J Allergy Clin Immunol 2011;127:145–152 - PubMed
-
- van Veen IH, Ten Brinke A, Gauw SA, Sterk PJ, Rabe KF, Bel EH. Consistency of sputum eosinophilia in difficult-to-treat asthma: a 5-year follow-up study. J Allergy Clin Immunol 2009;124:615–617 - PubMed
-
- Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax 2010;65:384–390 - PubMed
-
- Gibson PG. Inflammatory phenotypes in adult asthma: clinical applications. Clin Respir J 2009;3:198–206 - PubMed
-
- O'Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol 2011;128:257–263 - PubMed
Publication types
MeSH terms
Grants and funding
- U10 HL074227/HL/NHLBI NIH HHS/United States
- HL080414/HL/NHLBI NIH HHS/United States
- U10 HL074204/HL/NHLBI NIH HHS/United States
- HL097591/HL/NHLBI NIH HHS/United States
- HL090982/HL/NHLBI NIH HHS/United States
- R01 HL097591/HL/NHLBI NIH HHS/United States
- U10 HL074231/HL/NHLBI NIH HHS/United States
- U10 HL074225/HL/NHLBI NIH HHS/United States
- U10 HL074073/HL/NHLBI NIH HHS/United States
- U10 HL074212/HL/NHLBI NIH HHS/United States
- R01 HL080414/HL/NHLBI NIH HHS/United States
- U10 HL074206/HL/NHLBI NIH HHS/United States
- A1077439/PHS HHS/United States
- R01 HL090982/HL/NHLBI NIH HHS/United States
- U10 HL074208/HL/NHLBI NIH HHS/United States
- U10 HL074218/HL/NHLBI NIH HHS/United States

