Two alanine aminotranferases link mitochondrial glycolate oxidation to the major photorespiratory pathway in Arabidopsis and rice
- PMID: 22268146
- PMCID: PMC3346230
- DOI: 10.1093/jxb/err453
Two alanine aminotranferases link mitochondrial glycolate oxidation to the major photorespiratory pathway in Arabidopsis and rice
Abstract
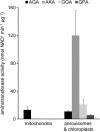
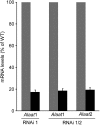
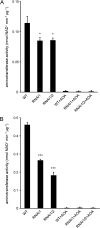
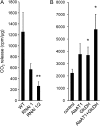
The major photorespiratory pathway in higher plants is distributed over chloroplasts, mitochondria, and peroxisomes. In this pathway, glycolate oxidation takes place in peroxisomes. It was previously suggested that a mitochondrial glycolate dehydrogenase (GlcDH) that was conserved from green algae lacking leaf-type peroxisomes contributes to photorespiration in Arabidopsis thaliana. Here, the identification of two Arabidopsis mitochondrial alanine:glyoxylate aminotransferases (ALAATs) that link glycolate oxidation to glycine formation are described. By this reaction, the mitochondrial side pathway produces glycine from glyoxylate that can be used in the glycine decarboxylase (GCD) reaction of the major pathway. RNA interference (RNAi) suppression of mitochondrial ALAAT did not result in major changes in metabolite pools under standard conditions or enhanced photorespiratroy flux, respectively. However, RNAi lines showed reduced photorespiratory CO(2) release and a lower CO(2) compensation point. Mitochondria isolated from RNAi lines are incapable of converting glycolate to CO(2), whereas simultaneous overexpression of GlcDH and ALAATs in transiently transformed tobacco leaves enhances glycolate conversion. Furthermore, analyses of rice mitochondria suggest that the side pathway for glycolate oxidation and glycine formation is conserved in monocotyledoneous plants. It is concluded that the photorespiratory pathway from green algae has been functionally conserved in higher plants.
Figures




Similar articles
-
Mitochondrial glycolate oxidation contributes to photorespiration in higher plants.J Exp Bot. 2007;58(10):2709-15. doi: 10.1093/jxb/erm131. Epub 2007 Jun 26. J Exp Bot. 2007. PMID: 17595195
-
A glycolate dehydrogenase in the mitochondria of Arabidopsis thaliana.J Exp Bot. 2004 Mar;55(397):623-30. doi: 10.1093/jxb/erh079. Epub 2004 Feb 13. J Exp Bot. 2004. PMID: 14966218
-
Photorespiration: current status and approaches for metabolic engineering.Curr Opin Plant Biol. 2010 Jun;13(3):249-56. doi: 10.1016/j.pbi.2010.01.006. Epub 2010 Feb 23. Curr Opin Plant Biol. 2010. PMID: 20185358 Review.
-
Two plastidic glycolate/glycerate translocator 1 isoforms function together to transport photorespiratory glycolate and glycerate in rice chloroplasts.J Exp Bot. 2021 Mar 29;72(7):2584-2599. doi: 10.1093/jxb/erab020. J Exp Bot. 2021. PMID: 33483723
-
Photorespiratory glycolate-glyoxylate metabolism.J Exp Bot. 2016 May;67(10):3041-52. doi: 10.1093/jxb/erw090. Epub 2016 Mar 19. J Exp Bot. 2016. PMID: 26994478 Review.
Cited by
-
New Insight into Aspartate Metabolic Pathways in Populus: Linking the Root Responsive Isoenzymes with Amino Acid Biosynthesis during Incompatible Interactions of Fusarium solani.Int J Mol Sci. 2022 Jun 7;23(12):6368. doi: 10.3390/ijms23126368. Int J Mol Sci. 2022. PMID: 35742809 Free PMC article.
-
The Arabidopsis thaliana gene annotated by the locus tag At3g08860 encodes alanine aminotransferase.Plant Direct. 2019 Sep 18;3(9):e00171. doi: 10.1002/pld3.171. eCollection 2019 Sep. Plant Direct. 2019. PMID: 31549019 Free PMC article.
-
Synthesis of β-Alanine From Isoleucine and Propionate Catabolism via Aminotransferases.Plant Direct. 2024 Dec 18;8(12):e70030. doi: 10.1002/pld3.70030. eCollection 2024 Dec. Plant Direct. 2024. PMID: 39703930 Free PMC article.
-
Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1392-400. doi: 10.1073/pnas.1424840112. Epub 2015 Feb 2. Proc Natl Acad Sci U S A. 2015. PMID: 25646482 Free PMC article.
-
The mitochondrial sulfur dioxygenase ETHYLMALONIC ENCEPHALOPATHY PROTEIN1 is required for amino acid catabolism during carbohydrate starvation and embryo development in Arabidopsis.Plant Physiol. 2014 May;165(1):92-104. doi: 10.1104/pp.114.239764. Epub 2014 Apr 1. Plant Physiol. 2014. PMID: 24692429 Free PMC article.
References
-
- Atkin OK, Evans JR, Siebke K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Australian Journal of Plant Physiology. 1998;25:437–443.
-
- Bari R, Kebeish R, Kalamajka R, Rademacher T, Peterhänsel C. A glycolate dehydrogenase in the mitochondria of Arabidopsis thaliana. Journal of Experimental Botany. 2004;55:623–630. - PubMed
-
- Bowles D, Lim EK, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophilic small molecules. Annual Review of Plant Biology. 2006;57:567–597. - PubMed
-
- Carrie C, Kühn K, Murcha MW, Duncan O, Small ID, O’Toole N, Whelan J. Approaches to defining dual-targeted proteins in Arabidopsis. The Plant Journal. 2009;57:1128–1139. - PubMed

