Evolving nonapeptide mechanisms of gregariousness and social diversity in birds
- PMID: 22269661
- PMCID: PMC3312996
- DOI: 10.1016/j.yhbeh.2012.01.005
Evolving nonapeptide mechanisms of gregariousness and social diversity in birds
Abstract
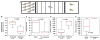
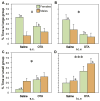
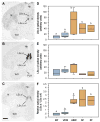
Of the major vertebrate taxa, Class Aves is the most extensively studied in relation to the evolution of social systems and behavior, largely because birds exhibit an incomparable balance of tractability, diversity, and cognitive complexity. In addition, like humans, most bird species are socially monogamous, exhibit biparental care, and conduct most of their social interactions through auditory and visual modalities. These qualities make birds attractive as research subjects, and also make them valuable for comparative studies of neuroendocrine mechanisms. This value has become increasingly apparent as more and more evidence shows that social behavior circuits of the basal forebrain and midbrain are deeply conserved (from an evolutionary perspective), and particularly similar in birds and mammals. Among the strongest similarities are the basic structures and functions of avian and mammalian nonapeptide systems, which include mesotocin (MT) and arginine vasotocin (VT) systems in birds, and the homologous oxytocin (OT) and vasopressin (VP) systems, respectively, in mammals. We here summarize these basic properties, and then describe a research program that has leveraged the social diversity of estrildid finches to gain insights into the nonapeptide mechanisms of grouping, a behavioral dimension that is not experimentally tractable in most other taxa. These studies have used five monogamous, biparental finch species that exhibit group sizes ranging from territorial male-female pairs to large flocks containing hundreds or thousands of birds. The results provide novel insights into the history of nonapeptide functions in amniote vertebrates, and yield remarkable clarity on the nonapeptide biology of dinosaurs and ancient mammals. This article is part of a Special Issue entitled Oxytocin, Vasopressin, and Social Behavior.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

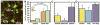
Similar articles
-
Mesotocin and nonapeptide receptors promote estrildid flocking behavior.Science. 2009 Aug 14;325(5942):862-6. doi: 10.1126/science.1174929. Science. 2009. PMID: 19679811 Free PMC article.
-
Neuropeptide regulation of social behavior in a monogamous cichlid fish.Physiol Behav. 2011 Mar 1;102(3-4):296-303. doi: 10.1016/j.physbeh.2010.11.022. Epub 2010 Nov 26. Physiol Behav. 2011. PMID: 21112347
-
Nonapeptides and social behavior in fishes.Horm Behav. 2012 Mar;61(3):230-8. doi: 10.1016/j.yhbeh.2011.12.016. Epub 2012 Jan 20. Horm Behav. 2012. PMID: 22285647 Review.
-
The formation and maintenance of social relationships increases nonapeptide mRNA in zebra finches of both sexes.Behav Neurosci. 2014 Feb;128(1):61-70. doi: 10.1037/a0035416. Behav Neurosci. 2014. PMID: 24512066
-
Deconstructing sociality, social evolution and relevant nonapeptide functions.Psychoneuroendocrinology. 2013 Apr;38(4):465-78. doi: 10.1016/j.psyneuen.2012.12.005. Epub 2013 Jan 4. Psychoneuroendocrinology. 2013. PMID: 23290368 Review.
Cited by
-
Variation in the Oxytocin Receptor Gene Predicts Brain Region-Specific Expression and Social Attachment.Biol Psychiatry. 2016 Jul 15;80(2):160-169. doi: 10.1016/j.biopsych.2015.12.008. Epub 2015 Dec 15. Biol Psychiatry. 2016. PMID: 26893121 Free PMC article.
-
Is Oxytocin "Nature's Medicine"?Pharmacol Rev. 2020 Oct;72(4):829-861. doi: 10.1124/pr.120.019398. Pharmacol Rev. 2020. PMID: 32912963 Free PMC article. Review.
-
A role for nonapeptides and dopamine in nest-building behaviour.J Neuroendocrinol. 2015 Feb;27(2):158-65. doi: 10.1111/jne.12250. J Neuroendocrinol. 2015. PMID: 25514990 Free PMC article.
-
Species, sex and individual differences in the vasotocin/vasopressin system: relationship to neurochemical signaling in the social behavior neural network.Front Neuroendocrinol. 2015 Jan;36:49-71. doi: 10.1016/j.yfrne.2014.07.001. Epub 2014 Aug 4. Front Neuroendocrinol. 2015. PMID: 25102443 Free PMC article. Review.
-
Emotions and courtship help bonded pairs cooperate, but emotional agents are vulnerable to deceit.Proc Natl Acad Sci U S A. 2023 Nov 14;120(46):e2308911120. doi: 10.1073/pnas.2308911120. Epub 2023 Nov 10. Proc Natl Acad Sci U S A. 2023. PMID: 37948585 Free PMC article.
References
-
- Absil P, Papello M, Viglietti-Panzica C, Balthazart J, Panzica G. The medial preoptic nucleus receives vasotocinergic inputs in male quail: a tract-tracing and immunocytochemical study. J Chem Neuroanat. 2002;24:27–39. - PubMed
-
- Acharjee S, Do-Rego JL, Oh da Y, Ahn RS, Choe H, Vaudry H, Kim K, Seong JY, Kwon HB. Identification of amino acid residues that direct differential ligand selectivity of mammalian and nonmammalian V1a type receptors for arginine vasopressin and vasotocin. Insights into molecular coevolution of V1a type receptors and their ligands. J Biol Chem. 2004;279:54445–54453. - PubMed
-
- Acher R. Endocrinology. American Physiological Society; Washington, D.C: 1972. Chemistry of the neurohypophysial hormones: an example of molecular evolution; pp. 119–130.
-
- Acher R, Chauvert J. The neurohypophysial endocrine regulatory cascade: Precursors, mediators, receptors, and effectors. Front Neuroendocrinol. 1995;16:237–289. - PubMed
-
- Adkins-Regan E. Neuroendocrine contributions to sexual partner preference in birds. Front Neuroendocrinol. 2011;32:155–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

