Unmasking the mysteries of the habenula in pain and analgesia
- PMID: 22270045
- PMCID: PMC3465722
- DOI: 10.1016/j.pneurobio.2012.01.004
Unmasking the mysteries of the habenula in pain and analgesia
Abstract
The habenula is a small bilateral structure in the posterior-medial aspect of the dorsal thalamus that has been implicated in a remarkably wide range of behaviors including olfaction, ingestion, mating, endocrine and reward function, pain and analgesia. Afferent connections from forebrain structures send inputs to the lateral and medial habenula where efferents are mainly projected to brainstem regions that include well-known pain modulatory regions such as the periaqueductal gray and raphe nuclei. A convergence of preclinical data implicates the region in multiple behaviors that may be considered part of the pain experience including a putative role in pain modulation, affective, and motivational processes. The habenula seems to play a role as an evaluator, acting as a major point of convergence where external stimuli is received, evaluated, and redirected for motivation of appropriate behavioral response. Here, we review the role of the habenula in pain and analgesia, consider its potential role in chronic pain, and review more recent clinical and functional imaging data of the habenula from animals and humans. Even through the habenula is a small brain structure, advances in structural and functional imaging in humans should allow for further advancement of our understanding of its role in pain and analgesia.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
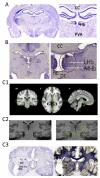
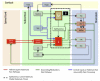
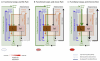
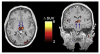

Similar articles
-
Mapping pain activation and connectivity of the human habenula.J Neurophysiol. 2012 May;107(10):2633-48. doi: 10.1152/jn.00012.2012. Epub 2012 Feb 8. J Neurophysiol. 2012. PMID: 22323632 Free PMC article.
-
Efferent pathways of the mouse lateral habenula.J Comp Neurol. 2015 Jan 1;523(1):32-60. doi: 10.1002/cne.23662. Epub 2014 Aug 30. J Comp Neurol. 2015. PMID: 25099741 Free PMC article.
-
Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): an indocarbocyanine dye (DiI) study.J Comp Neurol. 1996 Sep 2;372(4):529-43. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. J Comp Neurol. 1996. PMID: 8876451
-
Untangling the dorsal diencephalic conduction system: a review of structure and function of the stria medullaris, habenula and fasciculus retroflexus.Brain Struct Funct. 2020 Jun;225(5):1437-1458. doi: 10.1007/s00429-020-02069-8. Epub 2020 May 4. Brain Struct Funct. 2020. PMID: 32367265 Review.
-
Pain Modulation and the Transition from Acute to Chronic Pain.Adv Exp Med Biol. 2016;904:105-15. doi: 10.1007/978-94-017-7537-3_8. Adv Exp Med Biol. 2016. PMID: 26900066 Review.
Cited by
-
Psychotherapy in pain management: New viewpoints and treatment targets based on a brain theory.AIMS Neurosci. 2020 Jul 14;7(3):194-207. doi: 10.3934/Neuroscience.2020013. eCollection 2020. AIMS Neurosci. 2020. PMID: 32995484 Free PMC article.
-
Altered resting-state functional connectivity and effective connectivity of the habenula in irritable bowel syndrome: A cross-sectional and machine learning study.Hum Brain Mapp. 2020 Sep;41(13):3655-3666. doi: 10.1002/hbm.25038. Epub 2020 Jun 3. Hum Brain Mapp. 2020. PMID: 32488929 Free PMC article.
-
Circuits and functions of the lateral habenula in health and in disease.Nat Rev Neurosci. 2020 May;21(5):277-295. doi: 10.1038/s41583-020-0292-4. Epub 2020 Apr 8. Nat Rev Neurosci. 2020. PMID: 32269316 Review.
-
A systems-neuroscience model of phasic dopamine.Psychol Rev. 2020 Nov;127(6):972-1021. doi: 10.1037/rev0000199. Epub 2020 Jun 11. Psychol Rev. 2020. PMID: 32525345 Free PMC article.
-
Increased habenular connectivity in opioid users is associated with an α5 subunit nicotinic receptor genetic variant.Am J Addict. 2017 Oct;26(7):751-759. doi: 10.1111/ajad.12607. Epub 2017 Aug 31. Am J Addict. 2017. PMID: 28857330 Free PMC article.
References
-
- Agetsuma M, Aizawa H, Aoki T, Nakayama R, Takahoko M, Goto M, Sassa T, Amo R, Shiraki T, Kawakami K, Hosoya T, Higashijima S, Okamoto H. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010;13:1354–1356. - PubMed
-
- Alenina N, Bashammakh S, Bader M. Specification and differentiation of serotonergic neurons. Stem Cell Rev. 2006;2:5–10. - PubMed
-
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. - PubMed
-
- Andersen ERB, Dafney N. Electrophysiological evidence of concurrent dorsal raphe input to caudate, septum, habenula, thalamus hippocampus, cerebellum and olfactory bulb. Int. J. Neurosci. 1983;18:107–115. - PubMed
-
- Archer DP, Froelich J, McHugh M, Pappius HM. Local cerebral glucose utilization in stimulated rats sedated with thiopental. Anesthesiology. 1995;83:160–168. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

