The role of the N-domain in the ATPase activity of the mammalian AAA ATPase p97/VCP
- PMID: 22270372
- PMCID: PMC3318706
- DOI: 10.1074/jbc.M111.302778
The role of the N-domain in the ATPase activity of the mammalian AAA ATPase p97/VCP
Abstract
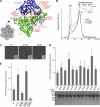
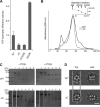
p97/valosin-containing protein (VCP) is a type II ATPase associated with various cellular activities that forms a homohexamer with each protomer containing an N-terminal domain (N-domain); two ATPase domains, D1 and D2; and a disordered C-terminal region. Little is known about the role of the N-domain or the C-terminal region in the p97 ATPase cycle. In the p97-associated human disease inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia, the majority of missense mutations are located at the N-domain D1 interface. Structure-based predictions suggest that such mutations affect the interaction of the N-domain with D1. Here we have tested ten major inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia-linked mutants for ATPase activity and found that all have increased activity over the wild type, with one mutant, p97(A232E), having three times higher activity. Further mutagenesis of p97(A232E) shows that the increase in ATPase activity is mediated through D2 and requires both the N-domain and a flexible ND1 linker. A disulfide mutation that locks the N-domain to D1 in a coplanar position reversibly abrogates ATPase activity. A cryo-EM reconstruction of p97(A232E) suggests that the N-domains are flexible. Removal of the C-terminal region also reduces ATPase activity. Taken together, our data suggest that the conformation of the N-domain in relation to the D1-D2 hexamer is directly linked to ATP hydrolysis and that the C-terminal region is required for hexamer stability. This leads us to propose a model where the N-domain adopts either of two conformations: a flexible conformation compatible with ATP hydrolysis or a coplanar conformation that is inactive.
Figures

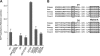

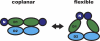
Similar articles
-
Hereditary inclusion body myopathy-linked p97/VCP mutations in the NH2 domain and the D1 ring modulate p97/VCP ATPase activity and D2 ring conformation.Mol Cell Biol. 2009 Aug;29(16):4484-94. doi: 10.1128/MCB.00252-09. Epub 2009 Jun 8. Mol Cell Biol. 2009. PMID: 19506019 Free PMC article.
-
A novel ATP-dependent conformation in p97 N-D1 fragment revealed by crystal structures of disease-related mutants.EMBO J. 2010 Jul 7;29(13):2217-29. doi: 10.1038/emboj.2010.104. Epub 2010 May 28. EMBO J. 2010. PMID: 20512113 Free PMC article.
-
D1 ring is stable and nucleotide-independent, whereas D2 ring undergoes major conformational changes during the ATPase cycle of p97-VCP.J Biol Chem. 2003 Aug 29;278(35):32784-93. doi: 10.1074/jbc.M303869200. Epub 2003 Jun 13. J Biol Chem. 2003. PMID: 12807884
-
Valosin-Containing Protein (VCP)/p97 Oligomerization.Subcell Biochem. 2024;104:485-501. doi: 10.1007/978-3-031-58843-3_18. Subcell Biochem. 2024. PMID: 38963497 Review.
-
ATP-bound form of the D1 AAA domain inhibits an essential function of Cdc48p/p97.Biochem Cell Biol. 2010 Feb;88(1):109-17. doi: 10.1139/o09-116. Biochem Cell Biol. 2010. PMID: 20130684 Review.
Cited by
-
Mechanism of allosteric inhibition of human p97/VCP ATPase and its disease mutant by triazole inhibitors.Commun Chem. 2024 Aug 9;7(1):177. doi: 10.1038/s42004-024-01267-3. Commun Chem. 2024. PMID: 39122922 Free PMC article.
-
VCP/p97 inhibitor CB-5083 modulates muscle pathology in a mouse model of VCP inclusion body myopathy.J Transl Med. 2022 Jan 8;20(1):21. doi: 10.1186/s12967-021-03186-6. J Transl Med. 2022. PMID: 34998409 Free PMC article.
-
Altered intersubunit communication is the molecular basis for functional defects of pathogenic p97 mutants.J Biol Chem. 2013 Dec 20;288(51):36624-35. doi: 10.1074/jbc.M113.488924. Epub 2013 Nov 6. J Biol Chem. 2013. PMID: 24196964 Free PMC article.
-
Lysine Methylation of the Valosin-Containing Protein (VCP) Is Dispensable for Development and Survival of Mice.PLoS One. 2015 Nov 6;10(11):e0141472. doi: 10.1371/journal.pone.0141472. eCollection 2015. PLoS One. 2015. PMID: 26544960 Free PMC article.
-
Dsc E3 ligase localization to the Golgi requires the ATPase Cdc48 and cofactor Ufd1 for activation of sterol regulatory element-binding protein in fission yeast.J Biol Chem. 2017 Sep 29;292(39):16333-16350. doi: 10.1074/jbc.M117.802025. Epub 2017 Aug 18. J Biol Chem. 2017. PMID: 28821619 Free PMC article.
References
-
- Bays N. W., Hampton R. Y. (2002) Cdc48-Ufd1-Npl4. Stuck in the middle with Ub. Curr. Biol. 12, R366–371 - PubMed
-
- Rape M., Hoppe T., Gorr I., Kalocay M., Richly H., Jentsch S. (2001) Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107, 667–677 - PubMed
-
- Hetzer M., Meyer H. H., Walther T. C., Bilbao-Cortes D., Warren G., Mattaj I. W. (2001) Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat. Cell Biol. 3, 1086–1091 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

