Favorable response to antigonadal therapy for a benign metastasizing leiomyoma
- PMID: 22270431
- PMCID: PMC3266540
- DOI: 10.1097/AOG.0b013e318240090e
Favorable response to antigonadal therapy for a benign metastasizing leiomyoma
Abstract
Background: Benign metastasizing leiomyoma and lymphangioleiomyomatosis (LAM) both are characterized by abnormal proliferation of smooth muscle-like cells in the lung.
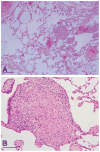
Case: A 32-year-old African woman with a diagnosis of LAM underwent myomectomy for uterine leiomyomas. An alternative diagnosis of benign metastasizing leiomyoma was made on repeat lung biopsy. Treatment with leuprolide acetate decreased pulmonary infiltrates and improved lung function and exercise tolerance.
Conclusion: Accurately diagnosing benign metastasizing leiomyoma has important implications for clinical outcome. Because its clinical presentation may be misleading, immunohistochemical techniques may assist in differentiating benign metastasizing leiomyoma from LAM. This is important because, in benign metastasizing leiomyoma, reduced tumor burden and improved pulmonary function may be achieved by suppressing gonadal steroids.
Figures



References
-
- Tietze L, Gunther K, Horbe A, et al. Benign metastasizing leiomyoma: a cytogenetically balanced but clonal disease. Hum Pathol. 2000;31:126–28. - PubMed
-
- Kayser K, Zink S, Schneider T, Dienemann H, André S, Kaltner H, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000;437:284–92. - PubMed
-
- McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–16. - PubMed
-
- Wentling GK, Sevin BU, Geiger XJ, Bridges MD. Benign metastasizing leiomyoma responsive to megestrol: case report and review of the literature. Int J Gynecol Cancer. 2005;15:1213–17. - PubMed
-
- Pitts S, Oberstein EM, Glassberg MK. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific diseases? Clin Chest Med. 2004;25:343–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

