Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways
- PMID: 22270714
- PMCID: PMC11029674
- DOI: 10.1007/s00262-012-1200-1
Sensitivity of a novel model of mammary cancer stem cell-like cells to TNF-related death pathways
Abstract
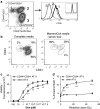
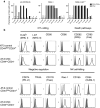
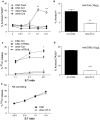
Cancer stem cells (CSC) are resistant to radiation and chemotherapy and play a significant role in cancer recurrence and metastatic disease. It is therefore important to identify alternative strategies, such as immunotherapies that can be used to control this refractory population. A CD44(+)CD24(-/low) subpopulation of cells within the B6 PyMT-MMTV transgenic mouse-derived AT-3 mammary carcinoma cell line was identified, which had CSC-like characteristics, including pluripotency and a resistance to chemo- and radiotherapy. Therefore, unlike xenograph models that require immunocompromised settings, this novel system may provide a means to study immune-mediated responses against CSC-like cells. The immunobiology of the AT-3 CSC-like cell population was studied by their surface molecule expression profile and their sensitivity to specified cell death pathways. Comparable levels of Rae-1, CD155, CD54 and higher levels of Fas and DR5 were expressed on the AT-3 CSC-like cells compared to non-CSC-like tumor cells. Expression correlated with an in vitro sensitivity to cell death by NK cells or through the ligation of the death receptors (Fas or DR5), by their ligands or anti-Fas and anti-DR5 mAbs. Indeed, compared to the rest of the AT-3 tumor cells, the CD44(+)CD24(-/low) subpopulation of cells were more sensitive to both Fas- and TRAIL-mediated cell death pathways. Therefore, despite the refractory nature of CSC to other conventional therapies, these CSC-like cells were not inherently resistant to specified forms of immune-mediated cell death. These results encourage the continued investigation into immunotherapeutic strategies as a means of controlling breast CSC, particularly through their cell death pathways.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Amurensin G enhances the susceptibility to tumor necrosis factor-related apoptosis-inducing ligand-mediated cytotoxicity of cancer stem-like cells of HCT-15 cells.Cancer Sci. 2013 Dec;104(12):1632-9. doi: 10.1111/cas.12299. Epub 2013 Nov 12. Cancer Sci. 2013. PMID: 24118446 Free PMC article.
-
Metformin-induced preferential killing of breast cancer initiating CD44+CD24-/low cells is sufficient to overcome primary resistance to trastuzumab in HER2+ human breast cancer xenografts.Oncotarget. 2012 Apr;3(4):395-8. doi: 10.18632/oncotarget.488. Oncotarget. 2012. PMID: 22565037 Free PMC article.
-
Induction of metastatic cancer stem cells from the NK/LAK-resistant floating, but not adherent, subset of the UP-LN1 carcinoma cell line by IFN-γ.Lab Invest. 2011 Oct;91(10):1502-13. doi: 10.1038/labinvest.2011.91. Epub 2011 Jun 20. Lab Invest. 2011. PMID: 21691263
-
Modulation of death receptor pathways in oncology.Drugs Today (Barc). 2003;39 Suppl C:95-109. Drugs Today (Barc). 2003. PMID: 14988748 Review.
-
Breast cancer stem cells and intrinsic subtypes: controversies rage on.Curr Stem Cell Res Ther. 2009 Jan;4(1):50-60. doi: 10.2174/157488809787169110. Curr Stem Cell Res Ther. 2009. PMID: 19149630 Review.
Cited by
-
Regulation of TRAIL-receptor expression by the ubiquitin-proteasome system.Int J Mol Sci. 2014 Oct 14;15(10):18557-73. doi: 10.3390/ijms151018557. Int J Mol Sci. 2014. PMID: 25318057 Free PMC article. Review.
-
Cancer stem cells: perspectives for therapeutic targeting.Cancer Immunol Immunother. 2015 Jan;64(1):91-7. doi: 10.1007/s00262-014-1592-1. Epub 2014 Aug 8. Cancer Immunol Immunother. 2015. PMID: 25104304 Free PMC article. Review.
-
Immune evasion by cancer stem cells.Regen Ther. 2021 Mar 11;17:20-33. doi: 10.1016/j.reth.2021.02.006. eCollection 2021 Jun. Regen Ther. 2021. PMID: 33778133 Free PMC article. Review.
-
Photodynamic therapy regulates fate of cancer stem cells through reactive oxygen species.World J Stem Cells. 2020 Jul 26;12(7):562-584. doi: 10.4252/wjsc.v12.i7.562. World J Stem Cells. 2020. PMID: 32843914 Free PMC article. Review.
-
Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model.Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12006-11. doi: 10.1073/pnas.1307935110. Epub 2013 Jun 10. Proc Natl Acad Sci U S A. 2013. PMID: 23754388 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

