Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice
- PMID: 22270715
- PMCID: PMC11029775
- DOI: 10.1007/s00262-012-1206-8
Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice
Abstract
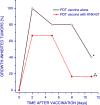
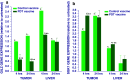
Photodynamic therapy (PDT)-generated cancer vaccines have shown promising results in preclinical studies and are being introduced in the clinics. Using an SCCVII mouse squamous cell carcinoma-based whole-cell autologous PDT vaccine model developed in our previous work, we have examined systemic effects in vaccinated mice that could be related to the induction of acute phase response. The upregulation of gene encoding serum amyloid P component (prototypic mouse acute phase reactant) was detected in the liver and to a lesser degree in the tumor of vaccinated mice at 24 h post-PDT vaccine treatment. A strong upregulation of gene for heat shock protein 70 was found in both the liver and tumor of mice at 4 h after their PDT vaccine treatment. Changes in the expression of genes for glucocorticoid-induced leucine zipper and serum- and glucocorticoid-regulated kinase 1 that are highly responsive to glucocorticoid modulation were uncovered in both the tumor and liver of vaccinated mice. A rise in the levels of serum corticosterone was detected in mice at 24 h after PDT vaccine treatment. The results indicate that a sudden appearance of a large number of PDT vaccine cells elicits host responses for securing their optimized clearance, which in addition to producing seminal acute phase reactants includes the engagement of glucocorticoid hormones. It is becoming increasingly clear that a consummate execution of this process of PDT vaccine cell removal is critical for tumor antigen recognition and the attainment of potent antitumor immune response.
Conflict of interest statement
The authors declare to have no conflict of interest in any form with respect to this article.
Figures




Similar articles
-
Photodynamic therapy-generated vaccine for cancer therapy.Cancer Immunol Immunother. 2006 Aug;55(8):900-9. doi: 10.1007/s00262-005-0088-4. Epub 2005 Oct 8. Cancer Immunol Immunother. 2006. PMID: 16215717 Free PMC article.
-
Expression of complement and pentraxin proteins in acute phase response elicited by tumor photodynamic therapy: the engagement of adrenal hormones.Int Immunopharmacol. 2010 Dec;10(12):1595-601. doi: 10.1016/j.intimp.2010.09.015. Epub 2010 Oct 8. Int Immunopharmacol. 2010. PMID: 20933626
-
Photodynamic therapy-generated vaccines: relevance of tumour cell death expression.Br J Cancer. 2007 Nov 19;97(10):1381-7. doi: 10.1038/sj.bjc.6604059. Epub 2007 Oct 30. Br J Cancer. 2007. PMID: 17971767 Free PMC article.
-
Acute phase response induction by cancer treatment with photodynamic therapy.Int J Cancer. 2008 Mar 15;122(6):1411-7. doi: 10.1002/ijc.23248. Int J Cancer. 2008. PMID: 18033689
-
PDT-associated host response and its role in the therapy outcome.Lasers Surg Med. 2006 Jun;38(5):500-8. doi: 10.1002/lsm.20337. Lasers Surg Med. 2006. PMID: 16634073 Review.
Cited by
-
Generation of an effective anti-lung cancer vaccine by DTPP-mediated photodynamic therapy and mechanistic studies.Lasers Med Sci. 2013 Sep;28(5):1383-92. doi: 10.1007/s10103-013-1270-0. Epub 2013 Feb 28. Lasers Med Sci. 2013. PMID: 23455655
-
Immunoregulatory Cell Depletion Improves the Efficacy of Photodynamic Therapy-Generated Cancer Vaccines.Int J Mol Sci. 2015 Nov 12;16(11):27005-14. doi: 10.3390/ijms161126008. Int J Mol Sci. 2015. PMID: 26569233 Free PMC article.
-
Calreticulin as cancer treatment adjuvant: combination with photodynamic therapy and photodynamic therapy-generated vaccines.Front Oncol. 2015 Feb 3;5:15. doi: 10.3389/fonc.2015.00015. eCollection 2015. Front Oncol. 2015. PMID: 25692097 Free PMC article.
-
A review and outlook in the treatment of osteosarcoma and other deep tumors with photodynamic therapy: from basic to deep.Oncotarget. 2017 Jun 13;8(24):39833-39848. doi: 10.18632/oncotarget.16243. Oncotarget. 2017. PMID: 28418855 Free PMC article. Review.
-
Prospects in the Application of Photodynamic Therapy in Oral Cancer and Premalignant Lesions.Cancers (Basel). 2016 Sep 2;8(9):83. doi: 10.3390/cancers8090083. Cancers (Basel). 2016. PMID: 27598202 Free PMC article. Review.
References
-
- Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer; an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. - DOI - PMC - PubMed
-
- Gollnick SO, Vaughan LA, Henderson BW. Generation of effective anti-tumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. - PubMed
-
- Korbelik M, Cecic I. Mechanism of tumor destruction by photodynamic therapy. In: Nalwa HS, editor. Handbook of photochemistry and photobiology. Stevenson Ranch: American Scientific Publishers; 2003. pp. 39–77.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

