Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2
- PMID: 22270964
- PMCID: PMC11824201
- DOI: 10.1007/s00432-011-1133-7
Estrogenic transmembrane receptor of GPR30 mediates invasion and carcinogenesis by endometrial cancer cell line RL95-2
Abstract
Purpose: The mechanisms underlying the effects of estrogen on endometrial cancer remain undefined. Although the classical mechanism of the action of estrogen involves binding to the estrogen receptors α and β, and transduction of the signal into the cell, G protein-coupled receptor (GPR) 30 has been shown to mediate nongenomic estrogen signaling. The goal of this study was to determine the role of GPR30 signal in the basic process such as invasion and carcinogenesis of endometrial cancer.
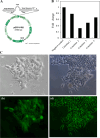
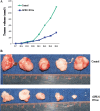
Methods: We downregulated the expression of GPR30 in endometrial cancer cell line RL95-2 by transfection with shGPR30-pGFP-V-RS, a GPR30 antisense expression vector. The cells were then subjected to an MTT assay and a Transwell(®) migration assay. And an animal model was also used to investigate the influence of downregulation of GPR30 on oncogenesis.
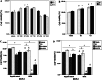
Results: Downregulation of GPR30 led to reduced growth and invasion by cells treated with 17β-estradiol. And the capacity of transfected RL95-2 cells to promote tumorigenesis was weakened in vivo.
Conclusions: Our data suggest that, for the endometrial cancer cell line RL95-2, GPR30 plays important roles in mediating the proliferative and invasive effects of estrogen and in tumorigenesis.
Figures

References
-
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Andò S, Maggiolini M (2007) G protein–coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res 67:1859–1866 - PubMed
-
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol 14:1649–1660 - DOI - PubMed
-
- Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E (2006) Distribution of GPR30, a Seven Membrane¨CSpanning estrogen receptor, in primary breast cancer and its association with clinicopathologic determinants of tumor progression. Clin Cancer Res 12:6359–6366 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

