Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983
- PMID: 22271474
- PMCID: PMC3295568
- DOI: 10.1200/JCO.2011.37.0171
Randomized phase III study comparing paclitaxel-bleomycin, etoposide, and cisplatin (BEP) to standard BEP in intermediate-prognosis germ-cell cancer: intergroup study EORTC 30983
Abstract
Purpose: To compare the efficacy of four cycles of paclitaxel-bleomycin, etoposide, and cisplatin (T-BEP) to four cycles of bleomycin, etoposide, and cisplatin (BEP) in previously untreated patients with intermediate-prognosis germ-cell cancer (GCC).
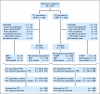
Patients and methods: Patients were randomly assigned to receive either T-BEP or standard BEP. Patients assigned to the T-BEP group received paclitaxel 175 mg/m(2) in a 3-hour infusion. Patients who were administered T-BEP received primary granulocyte colony-stimulating factor (G-CSF) prophylaxis. The study was designed as a randomized open-label phase II/III study. To show a 10% improvement in 3-year progression-free survival (PFS), the study aimed to recruit 498 patients but closed with 337 patients as a result of slow accrual.
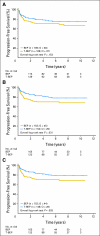
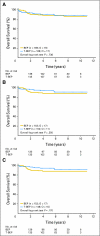
Results: Accrual was from November 1998 to April 2009. A total of 169 patients were administered BEP, and 168 patients were administered T-BEP. Thirteen patients in both arms were ineligible, mainly as a result of a good prognosis of GCC (eight patients administered BEP; six patients administered T-BEP) or a poor prognosis of GCC (one patient administered BEP; four patients administered T-BEP). PFS at 3 years (intent to treat) was 79.4% in the T-BEP group versus 71.1% in the BEP group (hazard ratio [HR], 0.73; CI, 0.47 to 1.13; P [log-rank test] = 0.153). PFS at 3 years in all eligible patients was 82.7% versus 70.1%, respectively (HR, 0.60; CI: 0.37 to 0.97) and was statistically significant (P = 0.03). Overall survival was not statistically different.
Conclusion: T-BEP administered with G-CSF seems to be a safe and effective treatment regimen for patients with intermediate-prognosis GCC. However, the study recruited a smaller-than-planned number of patients and included 7.7% ineligible patients. The primary analysis of the trial could not demonstrate statistical superiority of T-BEP for PFS. When ineligible patients were excluded, the analysis of all eligible patients demonstrated a 12% superior 3-year PFS with T-BEP, which was statistically significant.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Classical clinical trial design in testicular cancer: time to move on.J Clin Oncol. 2012 Mar 10;30(8):769-71. doi: 10.1200/JCO.2011.40.2503. Epub 2012 Jan 23. J Clin Oncol. 2012. PMID: 22271483 No abstract available.
Similar articles
-
Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial.Lancet Oncol. 2014 Dec;15(13):1442-1450. doi: 10.1016/S1470-2045(14)70490-5. Epub 2014 Nov 13. Lancet Oncol. 2014. PMID: 25456363 Free PMC article. Clinical Trial.
-
Paclitaxel+BEP (T-BEP) regimen as induction chemotherapy in poor prognosis patients with nonseminomatous germ cell tumors: a phase II study.Urology. 2011 Sep;78(3):620-5. doi: 10.1016/j.urology.2011.05.005. Epub 2011 Jul 20. Urology. 2011. PMID: 21764427 Clinical Trial.
-
A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974).Ann Oncol. 2011 May;22(5):1054-1061. doi: 10.1093/annonc/mdq575. Epub 2010 Nov 8. Ann Oncol. 2011. PMID: 21059637 Free PMC article. Clinical Trial.
-
[Testis germ cell tumours: which chemotherapy, for which patients?].Ann Urol (Paris). 2007 Apr;41(2):56-67. doi: 10.1016/j.anuro.2006.12.005. Ann Urol (Paris). 2007. PMID: 17486913 Review. French.
-
Treatment in germ cell tumours: state of the art.Eur J Surg Oncol. 1997 Apr;23(2):110-7. doi: 10.1016/s0748-7983(97)80002-9. Eur J Surg Oncol. 1997. PMID: 9158183 Review.
Cited by
-
The present and future of serum diagnostic tests for testicular germ cell tumours.Nat Rev Urol. 2016 Dec;13(12):715-725. doi: 10.1038/nrurol.2016.170. Epub 2016 Oct 18. Nat Rev Urol. 2016. PMID: 27754472 Review.
-
Paclitaxel, Ifosfamide, and Cisplatin Efficacy for First-Line Treatment of Patients With Intermediate- or Poor-Risk Germ Cell Tumors.J Clin Oncol. 2016 Jul 20;34(21):2478-83. doi: 10.1200/JCO.2016.66.7899. Epub 2016 May 16. J Clin Oncol. 2016. PMID: 27185842 Free PMC article. Clinical Trial.
-
Early experience with chemotherapy intensification for poor-prognosis metastatic germ cell cancer and unfavorable tumor marker decline.Can Urol Assoc J. 2020 Feb;14(2):43-47. doi: 10.5489/cuaj.5802. Epub 2019 Jul 23. Can Urol Assoc J. 2020. PMID: 31348750 Free PMC article.
-
Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial.Lancet Oncol. 2014 Dec;15(13):1442-1450. doi: 10.1016/S1470-2045(14)70490-5. Epub 2014 Nov 13. Lancet Oncol. 2014. PMID: 25456363 Free PMC article. Clinical Trial.
-
Improved outcomes in metastatic germ cell cancer: results from a large cohort study.J Cancer Res Clin Oncol. 2021 Feb;147(2):533-538. doi: 10.1007/s00432-020-03343-2. Epub 2020 Aug 9. J Cancer Res Clin Oncol. 2021. PMID: 32772232 Free PMC article.
References
-
- Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol. 1990;8:1777–1781. - PubMed
-
- International Germ Cell Cancer Collaborative Group (IGCCCG) International Germ Cell Consensus Classification: A prognostic-factor based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. - PubMed
-
- Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. - PubMed
-
- Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumor. J Clin Oncol. 2007;25:247–256. - PubMed
-
- Daugaard G, Skoneczna I, Aass N, et al. A randomized phase III study comparing standard dose BEP with sequential high-dose cisplatin, etoposide, and ifosfamide (VIP) plus stem-cell support in males with poor-prognosis germ-cell cancer. An intergroup study of EORTC, GTCSG, and Grupo Germinal (EORTC 30974) Ann Oncol. 2011;22:1054–1061. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2U10 CA011488-38/CA/NCI NIH HHS/United States
- 2U10 CA011488-33/CA/NCI NIH HHS/United States
- 2U10 CA011488-31/CA/NCI NIH HHS/United States
- 2U10 CA011488-34/CA/NCI NIH HHS/United States
- 2U10 CA011488-29/CA/NCI NIH HHS/United States
- 2U10 CA011488-39/CA/NCI NIH HHS/United States
- 2U10 CA011488-30/CA/NCI NIH HHS/United States
- 2U10 CA011488-28/CA/NCI NIH HHS/United States
- 2U10 CA011488-32/CA/NCI NIH HHS/United States
- U10 CA011488/CA/NCI NIH HHS/United States
- 2U10 CA011488-40/CA/NCI NIH HHS/United States
- 2U10 CA011488-35/CA/NCI NIH HHS/United States
- 2U10 CA011488-41/CA/NCI NIH HHS/United States
- 2U10 CA011488-37/CA/NCI NIH HHS/United States
- MC_U122861331/MRC_/Medical Research Council/United Kingdom
- 2U10 CA011488-36/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

