Scanning thin-sheet laser imaging microscopy elucidates details on mouse ear development
- PMID: 22271591
- PMCID: PMC5010664
- DOI: 10.1002/dvdy.23736
Scanning thin-sheet laser imaging microscopy elucidates details on mouse ear development
Erratum in
- Dev Dyn. 2013 May;242(5):591-2
Abstract
Background: The mammalian inner ear is transformed from a flat placode into a three-dimensional (3D) structure with six sensory epithelia that allow for the perception of sound and both linear and angular acceleration. While hearing and balance problems are typically considered to be adult onset diseases, they may arise as a developmental perturbation to the developing ear. Future prevention of hearing or balance loss requires an understanding of how closely genetic mutations in model organisms reflect the human case, necessitating an objective multidimensional comparison of mouse ears with human ears that have comparable mutations in the same gene.
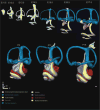
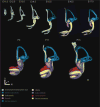
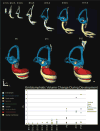
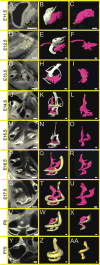
Results: Here, we present improved 3D analyses of normal murine ears during embryonic development using optical sections obtained through Thin-Sheet Laser Imaging Microscopy. We chronicle the transformation of an undifferentiated otic vesicle between mouse embryonic day 11.5 to a fully differentiated inner ear at postnatal day 15.
Conclusions: Our analysis of ear development provides new insights into ear development, enables unique perspectives into the complex development of the ear, and allows for the first full quantification of volumetric and linear aspects of ear growth. Our data provide the framework for future analysis of mutant phenotypes that are currently under-appreciated using only two dimensional renderings.
Copyright © 2012 Wiley Periodicals, Inc.
Figures





Similar articles
-
Gene transfer to the developing mouse inner ear by in vivo electroporation.J Vis Exp. 2012 Jun 30;(64):3653. doi: 10.3791/3653. J Vis Exp. 2012. PMID: 22781586 Free PMC article.
-
The role of Six1 in mammalian auditory system development.Development. 2003 Sep;130(17):3989-4000. doi: 10.1242/dev.00628. Development. 2003. PMID: 12874121 Free PMC article.
-
Developmental morphology of the mouse inner ear. A scanning electron microscopic observation.Acta Otolaryngol Suppl. 1985;422:1-69. Acta Otolaryngol Suppl. 1985. PMID: 3877398
-
Signaling regulating inner ear development: cell fate determination, patterning, morphogenesis, and defects.Congenit Anom (Kyoto). 2015 Feb;55(1):17-25. doi: 10.1111/cga.12072. Congenit Anom (Kyoto). 2015. PMID: 25040109 Review.
-
The development of the vertebrate inner ear.Mech Dev. 1998 Feb;71(1-2):5-21. doi: 10.1016/s0925-4773(97)00155-x. Mech Dev. 1998. PMID: 9507049 Review.
Cited by
-
Three-dimensional reconstructions from optical sections of thick mouse inner ears using confocal microscopy.J Microsc. 2012 Dec;248(3):292-8. doi: 10.1111/j.1365-2818.2012.03673.x. J Microsc. 2012. PMID: 23140378 Free PMC article.
-
Differentiation of Spiral Ganglion Neurons from Human Dental Pulp Stem Cells: A Further Step towards Autologous Auditory Nerve Recovery.Int J Mol Sci. 2024 Aug 22;25(16):9115. doi: 10.3390/ijms25169115. Int J Mol Sci. 2024. PMID: 39201803 Free PMC article.
-
Irx3/5 Null Deletion in Mice Blocks Cochlea-Saccule Segregation and Disrupts the Auditory Tonotopic Map.J Comp Neurol. 2024 Dec;532(12):e70008. doi: 10.1002/cne.70008. J Comp Neurol. 2024. PMID: 39655644 Free PMC article.
-
Continued expression of GATA3 is necessary for cochlear neurosensory development.PLoS One. 2013 Apr 16;8(4):e62046. doi: 10.1371/journal.pone.0062046. Print 2013. PLoS One. 2013. PMID: 23614009 Free PMC article.
-
Correct timing of proliferation and differentiation is necessary for normal inner ear development and auditory hair cell viability.Dev Dyn. 2013 Feb;242(2):132-47. doi: 10.1002/dvdy.23910. Dev Dyn. 2013. PMID: 23193000 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- R01 DC007588/DC/NIDCD NIH HHS/United States
- P30 DC010362/DC/NIDCD NIH HHS/United States
- R01-DC055095590/DC/NIDCD NIH HHS/United States
- UL1RR024979/RR/NCRR NIH HHS/United States
- UL1 RR024979/RR/NCRR NIH HHS/United States
- R21 DC005482/DC/NIDCD NIH HHS/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- DC007588-03S1/DC/NIDCD NIH HHS/United States
- TL1 RR024981/RR/NCRR NIH HHS/United States
- R01-DC007588/DC/NIDCD NIH HHS/United States
- R01 DC005590/DC/NIDCD NIH HHS/United States
- P30 DC 010362/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources

