CCR5 deficiency mitigates the deleterious effects of tumor necrosis factor α antagonism in murine histoplasmosis
- PMID: 22271672
- PMCID: PMC3282568
- DOI: 10.1093/infdis/jir869
CCR5 deficiency mitigates the deleterious effects of tumor necrosis factor α antagonism in murine histoplasmosis
Abstract
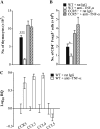
In murine histoplasmosis, tumor necrosis factor α (TNF-α) antagonism increases the number of regulatory T cells (Tregs) in lungs, and these cells profoundly hinder protective immunity. Because CCR5 mediates Treg homing and proliferation, we determined the outcome of antagonizing TNF-α in CCR5(-/)(-) mice infected with Histoplasma capsulatum. The absence of CCR5 attenuated the severity of infection associated with TNF-α neutralization. Infected controls given anti-TNF-α had a 10-fold increase in the number of Tregs in lungs compared with a <2-fold increase in CCR5(-/)(-) lungs. This difference was partially attributable to impaired homing in the absence of CCR5. Neutralization of TNF-α-enhanced CCR5 ligands in wild-type lungs thus promotes a gradient between lungs and the thymus. This study elucidates the interplay between TNF-α and CCR5 in histoplasmosis. The data suggest that targeting CCR5 may improve host immunity in individuals receiving TNF-α antagonists during infection.
Figures




Similar articles
-
Tumor Necrosis Factor Alpha Antagonism Reveals a Gut/Lung Axis That Amplifies Regulatory T Cells in a Pulmonary Fungal Infection.Infect Immun. 2018 May 22;86(6):e00109-18. doi: 10.1128/IAI.00109-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29581197 Free PMC article.
-
CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection.J Immunol. 2010 May 1;184(9):5224-31. doi: 10.4049/jimmunol.1000032. Epub 2010 Mar 24. J Immunol. 2010. PMID: 20335531 Free PMC article.
-
An aberrant thymus in CCR5-/- mice is coupled with an enhanced adaptive immune response in fungal infection.J Immunol. 2011 May 15;186(10):5949-55. doi: 10.4049/jimmunol.1003876. Epub 2011 Apr 8. J Immunol. 2011. PMID: 21478401 Free PMC article.
-
Resistance mechanisms in murine experimental histoplasmosis.Arch Med Res. 1993 Autumn;24(3):233-8. Arch Med Res. 1993. PMID: 7905306 Review.
-
The role of cytokines and chemokines in Histoplasma capsulatum infection.Cytokine. 2012 Apr;58(1):112-7. doi: 10.1016/j.cyto.2011.07.430. Epub 2011 Aug 26. Cytokine. 2012. PMID: 21871816 Free PMC article. Review.
Cited by
-
Tumor Necrosis Factor Alpha Antagonism Reveals a Gut/Lung Axis That Amplifies Regulatory T Cells in a Pulmonary Fungal Infection.Infect Immun. 2018 May 22;86(6):e00109-18. doi: 10.1128/IAI.00109-18. Print 2018 Jun. Infect Immun. 2018. PMID: 29581197 Free PMC article.
-
Mononuclear phagocyte-mediated antifungal immunity: the role of chemotactic receptors and ligands.Cell Mol Life Sci. 2015 Jun;72(11):2157-75. doi: 10.1007/s00018-015-1858-6. Epub 2015 Feb 26. Cell Mol Life Sci. 2015. PMID: 25715741 Free PMC article. Review.
-
Histoplasma Capsulatum: Mechanisms for Pathogenesis.Curr Top Microbiol Immunol. 2019;422:157-191. doi: 10.1007/82_2018_114. Curr Top Microbiol Immunol. 2019. PMID: 30043340 Free PMC article. Review.
References
-
- Zhou P, Sieve MC, Bennett J, et al. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–95. - PubMed
-
- Allendoerfer R, Deepe GS., Jr Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J Immunol. 2000;165:2657–64. - PubMed
-
- Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

