Alternative ferritin mRNA translation via internal initiation
- PMID: 22271759
- PMCID: PMC3285941
- DOI: 10.1261/rna.029322.111
Alternative ferritin mRNA translation via internal initiation
Erratum in
- RNA. 2012 Jun;18(6):1307
Abstract
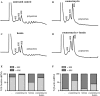
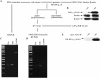
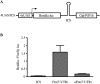
Ferritin stores and detoxifies an excess of intracellular iron, and thereby plays an important role in the metabolism of this metal. As unshielded iron promotes oxidative stress, ferritin is crucial in maintaining cellular redox balance and may also modulate cell growth, survival, and apoptosis. The expression of ferritin is controlled by transcriptional and post-transcriptional mechanisms. In light of the well-established transcriptional induction of ferritin by inflammatory signals, we examined how ferritin mRNA translation responds to stress conditions. We first used HT1080 fibrosarcoma cells engineered for coumermycin-inducible expression of PKR, a stress kinase that inhibits protein synthesis in virus-infected cells by phosphorylating eIF2α. In this setting, iron triggered partial ferritin mRNA translation despite a PKR-induced global shutdown in protein synthesis. Moreover, iron-mediated ferritin synthesis was evident in cells infected with an attenuated strain of poliovirus; when eIF4GI was cleaved, eIF2α was phosphorylated, and host protein synthesis was inhibited. Under global inhibition of protein synthesis or specific inhibition of ferritin mRNA translation in cells overexpressing PKR or IRP1, respectively, we demonstrate association of ferritin mRNA with heavy polysomes. Further experiments revealed that the 5' untranslated region (5' UTR) of ferritin mRNA contains a bona fide internal ribosomal entry site (IRES). Our data are consistent with the existence of an alternative, noncanonical mechanism for ferritin mRNA translation, which may primarily operate under stress conditions to protect cells from oxidative stress.
Figures


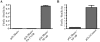

Similar articles
-
Conditional derepression of ferritin synthesis in cells expressing a constitutive IRP1 mutant.Mol Cell Biol. 2002 Jul;22(13):4638-51. doi: 10.1128/MCB.22.13.4638-4651.2002. Mol Cell Biol. 2002. PMID: 12052872 Free PMC article.
-
Ferritin mRNA translation, structure, and gene transcription during development of animals and plants.Enzyme. 1990;44(1-4):68-82. doi: 10.1159/000468748. Enzyme. 1990. PMID: 2133659
-
IRES-mediated translation of cellular messenger RNA operates in eIF2α- independent manner during stress.Nucleic Acids Res. 2012 Jan;40(2):541-52. doi: 10.1093/nar/gkr701. Epub 2011 Sep 14. Nucleic Acids Res. 2012. PMID: 21917851 Free PMC article.
-
Translational regulation by mRNA/protein interactions in eukaryotic cells: ferritin and beyond.Bioessays. 1993 Feb;15(2):85-90. doi: 10.1002/bies.950150203. Bioessays. 1993. PMID: 8471060 Review.
-
Translational regulation of ferritin synthesis by iron.Enzyme. 1990;44(1-4):42-58. doi: 10.1159/000468746. Enzyme. 1990. PMID: 2133657 Review.
Cited by
-
Chromatin balances cell redox and energy homeostasis.Epigenetics Chromatin. 2023 Nov 28;16(1):46. doi: 10.1186/s13072-023-00520-8. Epigenetics Chromatin. 2023. PMID: 38017471 Free PMC article. Review.
-
Protein synthesis control in cancer: selectivity and therapeutic targeting.EMBO J. 2022 Apr 19;41(8):e109823. doi: 10.15252/embj.2021109823. Epub 2022 Mar 22. EMBO J. 2022. PMID: 35315941 Free PMC article. Review.
-
Abnormal body iron distribution and erythropoiesis in a novel mouse model with inducible gain of iron regulatory protein (IRP)-1 function.J Mol Med (Berl). 2013 Jul;91(7):871-81. doi: 10.1007/s00109-013-1008-2. Epub 2013 Mar 1. J Mol Med (Berl). 2013. PMID: 23455710 Free PMC article.
-
Distinct phenotypes induced by acute hypoxia and TGF-β1 in human adult cardiac fibroblasts.J Mol Cell Cardiol Plus. 2024 Sep;9:100080. doi: 10.1016/j.jmccpl.2024.100080. Epub 2024 Jun 25. J Mol Cell Cardiol Plus. 2024. PMID: 39329164 Free PMC article.
-
Hydrogen peroxide induces La cytoplasmic shuttling and increases hepatitis C virus internal ribosome entry site-dependent translation.J Gen Virol. 2016 Sep;97(9):2301-2315. doi: 10.1099/jgv.0.000556. Epub 2016 Jul 18. J Gen Virol. 2016. PMID: 27436793 Free PMC article.
References
-
- Anderson P, Kedersha N 2008. Stress granules: the Tao of RNA triage. Trends Biochem Sci 33: 141–150 - PubMed
-
- Applegate LA, Scaletta C, Panizzon R, Frenk E 1998. Evidence that ferritin is UV inducible in human skin: part of a putative defense mechanism. J Invest Dermatol 111: 159–163 - PubMed
-
- Arosio P, Ingrassia R, Cavadini P 2009. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim Biophys Acta 1790: 589–599 - PubMed
-
- Baltzis D, Pluquet O, Papadakis AI, Kazemi S, Qu LK, Koromilas AE 2007. The eIF2α kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 282: 31675–31687 - PubMed
-
- Cairo G, Tacchini L, Pietrangelo A 1998. Lack of coordinate control of ferritin and transferrin receptor expression during rat liver regeneration. Hepatology 28: 173–178 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
