Changes in correlation between spontaneous activity of dorsal horn neurones lead to differential recruitment of inhibitory pathways in the cat spinal cord
- PMID: 22271870
- PMCID: PMC3413496
- DOI: 10.1113/jphysiol.2011.223271
Changes in correlation between spontaneous activity of dorsal horn neurones lead to differential recruitment of inhibitory pathways in the cat spinal cord
Abstract
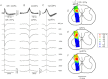
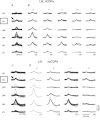
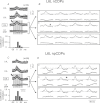
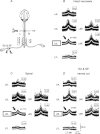
Simultaneous recordings of cord dorsum potentials along the lumbo-sacral spinal cord of the anaesthetized cat revealed the occurrence of spontaneous synchronous negative (n) and negative-positive (np) cord dorsum potentials (CDPs). The npCDPs, unlike the nCDPs, appeared preferentially associated with spontaneous negative dorsal root potentials (DRPs) resulting from primary afferent depolarization. Spontaneous npCDPs recorded in preparations with intact neuroaxis or after spinalization often showed a higher correlation than the nCDPs recorded from the same pair of segments. The acute section of the sural and superficial peroneal nerves further increased the correlation between paired sets of npCDPs and reduced the correlation between the nCDPs recorded from the same pair of segments. It is concluded that the spontaneous nCDPs and npCDPs are produced by the activation of interconnected sets of dorsal horn neurones located in Rexed's laminae III–IV and bilaterally distributed along the lumbo-sacral spinal cord. Under conditions of low synchronization in the activity of this network of neurones there would be a preferential activation of the intermediate nucleus interneurones mediating Ib non-reciprocal postsynaptic inhibition. Increased synchronization in the spontaneous activity of this ensemble of dorsal horn neurones would recruit the interneurones mediating primary afferent depolarization and presynaptic inhibition and, at the same time, reduce the activation of pathways mediating Ib postsynaptic inhibition. Central control of the synchronization in the spontaneous activity of dorsal horn neurones and its modulation by cutaneous inputs is envisaged as an effective mechanism for the selection of alternative inhibitory pathways during the execution of specific motor or sensory tasks.
Figures





Similar articles
-
Dynamic synchronization of ongoing neuronal activity across spinal segments regulates sensory information flow.J Physiol. 2015 May 15;593(10):2343-63. doi: 10.1113/jphysiol.2014.288134. Epub 2015 Mar 17. J Physiol. 2015. PMID: 25653206 Free PMC article.
-
Intersegmental synchronization of spontaneous activity of dorsal horn neurons in the cat spinal cord.Exp Brain Res. 2003 Feb;148(3):401-13. doi: 10.1007/s00221-002-1303-6. Epub 2002 Nov 28. Exp Brain Res. 2003. PMID: 12541150
-
Modulation of synaptic transmission from segmental afferents by spontaneous activity of dorsal horn spinal neurones in the cat.J Physiol. 2000 Dec 1;529 Pt 2(Pt 2):445-60. doi: 10.1111/j.1469-7793.2000.00445.x. J Physiol. 2000. PMID: 11101653 Free PMC article.
-
Interactions between pathways controlling posture and gait at the level of spinal interneurones in the cat.Prog Brain Res. 1993;97:161-71. doi: 10.1016/s0079-6123(08)62274-8. Prog Brain Res. 1993. PMID: 8234742 Review.
-
Selectivity of the central control of sensory information in the mammalian spinal cord.Adv Exp Med Biol. 2002;508:157-70. doi: 10.1007/978-1-4615-0713-0_19. Adv Exp Med Biol. 2002. PMID: 12171106 Review.
Cited by
-
Synchronous firing of dorsal horn neurons at the origin of dorsal root reflexes in naïve and paw-inflamed mice.Front Cell Neurosci. 2022 Sep 23;16:1004956. doi: 10.3389/fncel.2022.1004956. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36212688 Free PMC article.
-
Dynamic synchronization of ongoing neuronal activity across spinal segments regulates sensory information flow.J Physiol. 2015 May 15;593(10):2343-63. doi: 10.1113/jphysiol.2014.288134. Epub 2015 Mar 17. J Physiol. 2015. PMID: 25653206 Free PMC article.
-
Needling Interventions for Sciatica: Choosing Methods Based on Neuropathic Pain Mechanisms-A Scoping Review.J Clin Med. 2021 May 19;10(10):2189. doi: 10.3390/jcm10102189. J Clin Med. 2021. PMID: 34069357 Free PMC article.
-
Supraspinal Shaping of Adaptive Transitions in the State of Functional Connectivity Between Segmentally Distributed Dorsal Horn Neuronal Populations in Response to Nociception and Antinociception.Front Syst Neurosci. 2019 Sep 26;13:47. doi: 10.3389/fnsys.2019.00047. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31616259 Free PMC article.
-
Markovian Analysis of the Sequential Behavior of the Spontaneous Spinal Cord Dorsum Potentials Induced by Acute Nociceptive Stimulation in the Anesthetized Cat.Front Comput Neurosci. 2017 May 1;11:32. doi: 10.3389/fncom.2017.00032. eCollection 2017. Front Comput Neurosci. 2017. PMID: 28507514 Free PMC article.
References
-
- Beloozerova IN, Rossignol S. Antidromic discharges in dorsal roots of decerebrate cats. II: Studies during treadmill locomotion. Brain Res. 2004;996:227–236. - PubMed
-
- Biella G, Riva L, Sotgiu ML. Interaction between neurones in different laminae of the dorsal horn of the spinal cord. A correlation study in normal and neuropathic rats. Eur J Neurosci. 1997;9:1017–1025. - PubMed
-
- Biella G, Sotgiu ML. Evidence that inhibitory mechanisms mask inappropriate somatotopic connections in the spinal cord of normal rat. J Neurophysiol. 1995;74:495–505. - PubMed
-
- Chu J, Wagle-Shukla A, Gunraj C, Lang AE, Chen R. Impaired presynaptic inhibition in the motor cortex in Parkinson disease. Neurology. 2009;72:842–849. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

