Ubiquitin-proteasome-rich cytoplasmic structures in neutrophils of patients with Shwachman-Diamond syndrome
- PMID: 22271888
- PMCID: PMC3396678
- DOI: 10.3324/haematol.2011.048462
Ubiquitin-proteasome-rich cytoplasmic structures in neutrophils of patients with Shwachman-Diamond syndrome
Abstract
Background: Shwachman-Diamond syndrome is an autosomal recessive disorder in which severe bone marrow dysfunction causes neutropenia and an increased risk of leukemia. Recently, novel particulate cytoplasmic structures, rich in ubiquitinated and proteasomal proteins, have been detected in epithelial cells and neutrophils from patients with Helicobacter pylori gastritis and several epithelial neoplasms.
Design and methods: Blood neutrophils from 13 cases of Shwachman-Diamond syndrome - ten with and three without SBDS gene mutation - and ten controls were investigated by confocal microscopy and ultrastructural immunocytochemistry using antibodies against ubiquitinated proteins, proteasomes, p62 protein, and Helicobacter pylori VacA, urease and outer membrane proteins.
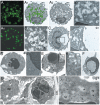
Results: Many extensively disseminated particulate cytoplasmic structures, accounting for 22.78 ± 5.57% (mean ± standard deviation) of the total cytoplasm, were found in blood neutrophils from mutated Shwachman-Diamond syndrome patients. The particulate cytoplasmic structures showed immunoreactivity for polyubiquitinated proteins and proteasomes, but no reactivity for Helicobacter pylori products, which are present in particulate cytoplasmic structures of Helicobacter pylori-positive gastritis. Neutrophils from patients with Shwachman-Diamond syndrome frequently showed p62-positive autophagic vacuoles and apoptotic changes in 5% of cells. No particulate cytoplasmic structures were observed in most control neutrophils; however, in a few cells from two cases we noted focal development of minute particulate cytoplasmic structures, accounting for 0.74 ± 0.56% of the total cytoplasm (P<0.001 versus particulate cytoplasmic structures from mutated Shwachman-Diamond syndrome patients). Neutrophils from non-mutated Shwachman-Diamond-syndrome-like patients resembled controls in two cases, and a third case showed particulate cytoplasmic structure patterns intermediate between those in controls and those in mutated Shwachman-Diamond syndrome patients.
Conclusions: Particulate cytoplasmic structures are a prominent feature of neutrophils from patients with Shwachman-Diamond syndrome. They may help us to understand the mechanism of granulocyte dysfunction and the neoplastic risk of the disease.
Figures

Similar articles
-
Dysplastic neutrophils in the bone marrow of a Shwachman-Diamond syndrome patient.Blood. 2017 Jul 6;130(1):96. doi: 10.1182/blood-2017-03-773846. Blood. 2017. PMID: 28684451 No abstract available.
-
Pregnancy in Shwachman-Diamond syndrome: a novel genetic mutation with minimal consequence.BMJ Case Rep. 2012 Nov 1;2012:bcr2012007305. doi: 10.1136/bcr-2012-007305. BMJ Case Rep. 2012. PMID: 23125299 Free PMC article.
-
The Greek Registry of Shwachman Diamond-Syndrome: Molecular and clinical data.Pediatr Blood Cancer. 2017 Nov;64(11). doi: 10.1002/pbc.26630. Epub 2017 May 16. Pediatr Blood Cancer. 2017. PMID: 28509441
-
[Research progress of pathogenic mechanism of congenital neutropenia].Zhonghua Er Ke Za Zhi. 2012 Nov;50(11):868-71. Zhonghua Er Ke Za Zhi. 2012. PMID: 23302623 Review. Chinese. No abstract available.
-
Diagnosis, Treatment, and Molecular Pathology of Shwachman-Diamond Syndrome.Hematol Oncol Clin North Am. 2018 Aug;32(4):687-700. doi: 10.1016/j.hoc.2018.04.006. Epub 2018 Jun 5. Hematol Oncol Clin North Am. 2018. PMID: 30047420 Review.
Cited by
-
Polyubiquitinated proteins, proteasome, and glycogen characterize the particle-rich cytoplasmic structure (PaCS) of neoplastic and fetal cells.Histochem Cell Biol. 2014 May;141(5):483-97. doi: 10.1007/s00418-014-1202-5. Epub 2014 Mar 1. Histochem Cell Biol. 2014. PMID: 24577783
-
PaCS is a novel cytoplasmic structure containing functional proteasome and inducible by cytokines/trophic factors.PLoS One. 2013 Dec 17;8(12):e82560. doi: 10.1371/journal.pone.0082560. eCollection 2013. PLoS One. 2013. PMID: 24358206 Free PMC article.
-
Chaperone molecules concentrate together with the ubiquitin-proteasome system inside particulate cytoplasmic structures: possible role in metabolism of misfolded proteins.Histochem Cell Biol. 2015 Aug;144(2):179-84. doi: 10.1007/s00418-015-1327-1. Epub 2015 May 8. Histochem Cell Biol. 2015. PMID: 25952156
-
Proteasome-Rich PaCS as an Oncofetal UPS Structure Handling Cytosolic Polyubiquitinated Proteins. In Vivo Occurrence, in Vitro Induction, and Biological Role.Int J Mol Sci. 2018 Sep 14;19(9):2767. doi: 10.3390/ijms19092767. Int J Mol Sci. 2018. PMID: 30223470 Free PMC article. Review.
-
Particulate cytoplasmic structures with high concentration of ubiquitin-proteasome accumulate in myeloid neoplasms.J Hematol Oncol. 2015 Jun 18;8:71. doi: 10.1186/s13045-015-0169-6. J Hematol Oncol. 2015. PMID: 26081257 Free PMC article.
References
-
- Rommens JM, Durie PR. Shwachman-Diamond Syndrome. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews. Seattle (WA): University of Washington, Seattle; 1993–2008. Jul 17, - PubMed
-
- Dror Y, Freedman Shwachman-Diamond syndrome marrow cells show abnormally increased apoptosis mediated through the Fas pathway. Blood. 2001;97(10):3011–6. - PubMed
-
- Boocock GR, Morrison JA, Popovic M, Richards N, Ellis N, Durie PR, et al. Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet. 2003;33(1):97–101. - PubMed
-
- Menne TF, Goyenechea B, Sanchez-Puig N, Wong CC, Tonkin LM, Ancliff PJ, et al. The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat Genet. 2007;39(4):486–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

