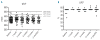Clinical cardiac safety profile of nilotinib
- PMID: 22271904
- PMCID: PMC3366654
- DOI: 10.3324/haematol.2011.058776
Clinical cardiac safety profile of nilotinib
Abstract
Background: Nilotinib is a second-generation tyrosine kinase inhibitor with significant efficacy as first- or second-line treatment in patients with chronic myeloid leukemia. Despite preclinical evidence indicating a risk of prolongation of the QT interval, which was confirmed in clinical trials, detailed information on nilotinib's cardiac safety profile is lacking.
Design and methods: Here, we retrospectively assessed cardiovascular risk factors in 81 patients who were being or had previously been treated with nilotinib therapy and evaluated cardiovascular parameters by longitudinal monitoring of the QT interval and left ventricular ejection fraction. Detailed information on the occurrence and management of defined cardiac adverse events was extracted.
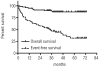
Results: The median duration of nilotinib therapy was 26 months (range, 1-72). The median QT interval at baseline was 413 msec (range, 368-499 msec). During follow-up, the median QT was not significantly different from the baseline value at any time-point. Sixteen of 81 patients (20%) had new electrocardiographic changes. Cardiac function, as assessed by measurement of left ventricular ejection fraction, did not change significantly from baseline at any time-point. During a median follow-up of 44 months (range, 2-73), seven patients (9%), all of whom had received prior imatinib therapy, developed 11 clinical cardiac adverse events requiring treatment. The median time from the start of nilotinib therapy to an event was 14.5 months (range, 2-68). Five of seven patients were able to continue nilotinib therapy with only one brief interruption.
Conclusions: Whereas new electrocardiographic abnormalities were recorded in 20% of all patients and some of them developed severe or even life-threatening coronary artery disease, QT prolongation, changes in left ventricular ejection fraction, and clinical cardiac adverse events were uncommon in patients treated with nilotinib.
Figures
References
-
- Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112(13):4808–17. - PubMed
-
- Hehlmann R, Hochhaus A, Baccarani M. Chronic myeloid leukaemia. Lancet. 2007;370(9584):342–50. - PubMed
-
- Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8(11):1018–29. - PubMed
-
- Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7(5):345–56. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical